| International Journal of Clinical Pediatrics, ISSN 1927-1255 print, 1927-1263 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Int J Clin Pediatr and Elmer Press Inc |
| Journal website https://ijcp.elmerpub.com |
Original Article
Volume 14, Number 2, October 2025, pages 44-50
Effect of Pediatric Hospitalist-Run Post-Discharge Care Clinic on Hospital Efficiency
Jessica Santuccia, d , Meghan Hartmana, Abigail Nelsonb, Tonya Kingc, Michael Beckb
aPenn State College of Medicine, Hershey, PA 17033, USA
bDepartment of Pediatrics, Division of Pediatric Hospital Medicine, Hershey Medical Center, Hershey, PA 17033, USA
cSerasota Memorial Hospital Research Institute, Sarasota, FL 34239, USA
dCorresponding Author: Jessica Santucci, Penn State College of Medicine, Hershey, PA 17033, USA
Manuscript submitted September 4, 2025, accepted October 7, 2025, published online October 17, 2025
Short title: Hospitalist Adoption of Care Transitions
doi: https://doi.org/10.14740/ijcp1026
| Abstract | ▴Top |
Background: A large number of pediatric patients requiring feeding tubes have historically remained in the hospital until they could tolerate oral feeds and medication, despite not needing continuous monitoring. The creation of post-discharge care clinics (PDCCs) to follow newborns discharged from neonatal intensive care units with nasoenteric feeding tubes promoted progress towards full oral feeds and cost savings without increasing adverse events. However, research focused on older pediatric populations is sparse. This study evaluates a pediatric hospitalist-run PDCC and its impact on inpatient length of stay (LOS), readmissions, emergency department (ED) visits, and cost reduction outcomes for patients discharged with nasoenteric feeding tubes.
Methods: A retrospective cohort of pediatric patients discharged with nasoenteric feeding tubes and followed up with the PDCC from July 2019 to July 2021 was conducted. Inclusion criteria for both groups involved patients ≤ 24 months old discharged from the hospitalist service, LOS > 24 h, and documented nasoenteric feeding orders. These patients were compared to controls who stayed hospitalized until they achieved full oral feeds. Fifty-seven PDCC and 62 control patients were analyzed using SAS version 9.4, for LOS, readmission rate, and ED visits.
Results: PDCC patients had significant median cost savings of $24,813.80 (P = 0.002) per patient. Median LOS was 6 days with 71.9% having no ED visits and 77.2% with no readmissions within 30 days post-discharge compared to a population without PDCC access.
Conclusions: This study demonstrates that the PDCC decreases inpatient LOS while limiting adverse outcomes to generate cost savings for patients. While showing promise in promoting improved quality and efficiency of care for discharged patients, future research should assess PDCC function in other pediatric populations.
Keywords: Transitions of care; Pediatric hospital medicine; NGT; Quality improvement
| Introduction | ▴Top |
Pediatric care has become increasingly regionalized, making children’s hospitals and their service lines responsible for providing care to a greater number of children [1]. This places children’s hospitals under immense pressure to optimize resource utilization, without decreasing quality or value of care. This requires creative efforts to reduce length of stay (LOS) without increasing hospital readmissions or encroaching on patient’s, staff satisfaction, or other key performance indicators [2-7]. Anticipating these demands, the Division of Pediatric Hospital Medicine started a post-discharge care clinic (PDCC) in 2015. Its focus was on providing wraparound services across the care continuum to patients who no longer meet inpatient criteria but have existing medical needs. The PDCC was designed to follow up on pending labs and/or diagnostic studies that could influence medical management and monitor for adverse events as well as follow uninsured patients and/or patients who did not have a primary care physician (PCP) at time of discharge. It was also structured to manage patients who were discharged from the hospital with new temporary technologies due to an acute illness (e.g. nasogastric tube (NGT) or nasojejunal tube (NJT), nasal cannula oxygen, or peripherally inserted central catheters). Prior to the PDCC’s existence, patients remained in the hospital to fully transition to oral feeds without an NGT/NJT, wean from supplemental oxygen, or complete intravenous antibiotic courses. By 2018, the clinic experienced enough growth that justified hiring a full-time registered nurse-case manager (RN-case manager). Together, the RN-case manager and hospitalist physician improved the PDCC’s capability to specifically follow patients with NGT/NJT as outpatients until they gain enough weight to remove the feeding tube or be seen in our pediatric feeding clinic.
Neonatologists have been using a dedicated clinic for the management of neonates discharged from the neonatal intensive care unit (NICU) with nasoenteral tubes for decades. Their data demonstrate excellent outcomes regarding time to full oral feedings, patient satisfaction, and significant cost savings [8-12]. A recent study of pediatric patients discharged with nasoenteral feeding tubes found that nearly half of the patients had an emergency department (ED) visit or hospital readmission 30 days following discharge [13]. Another study demonstrated improved growth and feeding outcomes for patients discharged with NGT/NJT, but they were followed by a pediatric gastroenterology clinic after being discharged from the hospital [9]. Neither of these studies assessed cost reduction.
Our study adds to this growing body of literature by investigating if similar outcomes can be achieved via a pediatric hospitalist-run clinic for patients who are discharged with nasoenteral tubes. We retrospectively examined the impact of the PDCC on several outcomes, including: 1) LOS, 2) cost reduction, and 3) readmissions or repeat ED visits.
| Materials and Methods | ▴Top |
This retrospective cohort analysis obtained data from a single 133-bed pediatric tertiary care children’s hospital in south-central Pennsylvania and was deemed exempt by the institutional review board secondary to the use of retrospective, de-identified clinical data (00020944). All procedures performed were in accordance with the institutional ethical standards.
Patients seen in the PDCC with an NGT/NJT between July 1, 2019 and July 1, 2021 were identified. Patients with “NGT or NJT management” listed as the rationale for their PDCC visit were pulled for evaluation. Based on the clinic’s specific code-based rationale, this encompassed patients who had a new NJT/NGT placed during their hospital admission. Patients requiring NGT/NJT often have malnutrition, oral aversions, aspiration risks, and/or dysphagia. The records of each patient were then manually reviewed to ensure that inclusion criteria were met. Author (JS) followed a standardized process for patient data extraction through PowerChart. JS retrieved facility identification numbers for each associated PDCC visit. JS then abstracted associated demographic and clinical data, such as LOS and International Classification of Diseases, 10th Revision (ICD-10) codes. Readmissions and ED visits 30 days post-discharge, achievement of full oral feeds, and financial data from inpatient and outpatient encounters were also pulled to gain comprehensive insight into each patient’s care experience. Author (AN) served as an adjudicator to review the data collected by JS. Author (MH) then followed the same standardized process to extract matching patient data for the control group. All ED visits and readmissions were assessed in relation to the same hospital.
Inclusion criteria for the PDCC group included: patients discharged from the pediatric hospitalist service between July 1, 2019 and July 1, 2021 with an NGT or NJT, patients ≤ 24 months old, patients admitted under inpatient status, those with an LOS > 24 h, and patients with nasoenteric insertion and care orders. Exclusion criteria involved children > 24 months old, patients with chronic neurological or respiratory conditions requiring gastrostomy tubes, and patients discharged from the NICU, pulmonary, gastroenterology, cardiology, hematology/oncology, or surgical services. The time range of the experimental group was chosen as 2019 - 2021 because a full-time RN-case manager was brought to the scene in 2019, and the clinic subsequently experienced growth in patient utilization. Therefore, this timeframe was used to ensure that an adequate sample size existed. The control group included children admitted to the pediatric hospital medicine service between the July 1, 2017 and July 1, 2019 with the same diagnoses, but opted to not receive follow-up care via the PDCC and were instead admitted to the inpatient service until achieving full oral feeds, thus adhering to the traditional standard of care. The timeframe of 2017 - 2019 was selected for the control group given the reality that these patients opted out of PDCC usage, and a full-time RN-case manager was not yet available to contribute to PDCC parental educational experience. These data included patients that had an LOS > 24 h, were discharged from the pediatric hospitalist service, were between the ages of 0 and 24 months (excluding NICU patients), had NGT/NJT orders, and had at least one of the top 10 ICD-10 codes from the PDCC clinic population.
Outcomes and study variables
LOS
Time stamped data were used to calculate LOS. The date and time of day the discharge order was placed was subtracted from the date and time the admission order was placed to get LOS. Given the small sample size, LOS is reported as median days as opposed to average LOS.
The estimate of inpatient LOS reduction for PDCC patients with the top 10 most common ICD-10 codes was the primary outcome measure, which were: delayed growth (R62.51), acute respiratory failure (J96.01), dehydration (E86.0), dysphagia (R13.10), gastro-esophageal reflux disease (K21.9), periodic breathing (R06.03), other specified health status (Z78.9), feeding difficulties (R63.3), other lack of expected normal physiological development (R63.39), and acute bronchiolitis due to respiratory syncytial virus (J21.0). Reduction in LOS was calculated by subtracting the days between discharge and removal of either the NGT or NJT. This method served as a proxy to reflect the historical practice of keeping children with NGT/NJT as an inpatient to achieve full oral feeds prior to the existence of the PDCC. ED visit and readmission rates 30 days post-discharge control data were derived from a similar patient population of n = 62, as described above. Secondary outcome measures were ED visits, readmissions, time taken to achieve full oral feeds, and cost savings obtained from the estimated LOS reduction.
ED visits and readmissions
The rates of ED visits and readmissions within 30 days post-discharge from the pediatric hospitalist service were compared to the control data.
Cost savings
Since LOS is a major cost driver, the estimated cost savings obtained through utilization of the PDCC were obtained by estimating the decrease in inpatient LOS for the above ICD-10 codes for PDCC patients. This estimate was calculated by obtaining the number of days taken for the patient to achieve full oral feeds or when the feeding tube was removed. If full oral feeds were not reached, the number of days passed until they were seen at the feeding clinic for potential gastrostomy-tube placement was used as a proxy for this outcome. Cost saved per day was obtained by dividing the total cost of each patient’s hospital stay on the day before discharge by the hospital LOS. This number was multiplied by the cost per day to produce an estimate of the total cost savings of patients seen in the PDCC. Of note, LOS estimation considered the days until patients were seen at the feeding clinic, but these patients were not used in analyzing time to full oral feeds, since they never ultimately achieved this goal.
Statistical analyses
Summary statistics were reported to describe the study sample, medians and quartiles for continuous measures and frequencies and percentages for categorical measures. The rates of 30-day ED revisit and readmissions in the PDCC sample were compared to controls using Chi-squared tests. Cost savings were estimated as defined above and reported with medians and quartiles, with the Kruskal-Wallis test used to compare median costs between groups. Statistical analyses were performed using SAS statistical software version 9.4 (SAS Institute Inc., Cary, NC). A value of P < 0.05 was considered statistically significant.
| Results | ▴Top |
Regarding the PDCC data, the initial search identified 74 patients. Following reviews conducted by JS and AN, 17 patients were excluded leaving 57 patients for analysis. Of the control group, 62 patients were identified and all met inclusion criteria, resulting in no exclusions.
Fifty-seven patients were seen by the PDCC between July 1, 2019 and July 1, 2021 with a median patient age of 2 months old (interquartile (IQR) 1 - 6 months) and LOS of 6 days (IQR 3 - 8 days). Most patients were white (67%), were established with a PCP (95%), and used English as a primary language (90%). The male-to-female ratio was almost equivalent as well. Patients had varying insurance providers, with the most common being Amerihealth Caritas (16%), Blue Cross Out of State (12%), and Aetna Better Health Kid (12%). The most common ICD-10 codes were failure to thrive (R62.51) in 20 patients (35%) and acute respiratory failure (J96.01) in 11 patients (19%). Eighteen percent of patients were admitted to the intensive care unit (Table 1). Manual abstraction of data revealed that only one patient (2%) did not make a follow-up appointment.
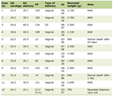 Click to view | Table 1. PDCC and Control Clinic Patient Demographics |
Of the 57 patients, 26 (51%) achieved full oral feeds, requiring a median of 24.5 days (IQR 7 - 55) to achieve this goal on an outpatient basis. Nine (15.8%) patients required gastrostomy tube placement after not reaching full oral feeds, and the remainder of the patients (35.2%) established with a feeding clinic for tube feed management. Forty-four patients received speech therapy, and the median number of days from discharge until outpatient speech therapy was started was 16 (IQR 8 - 44.5). Twenty-three patients had swallow assessments with 19 patients (83%) experiencing documented aspiration events. The median LOS for the control group was 10 days, while the median LOS for the PDCC group was 6 days. The median LOS for patients discharged from the PDCC (6 days) was then compared to the median number of days taken to achieve full oral feeds (10 days) to estimate that the PDCC saved patients an average 4 inpatient days. In terms of cost savings, each of the 26 patients that achieved full oral feeds had a median total savings of $24,813.80 (IQR $10,873.50 - $57,181.70). Kruskal-Wallis testing revealed that these savings were significant in comparison to the cost associated with the LOS seen in the control group, which demonstrated a median total cost of $5,435.10 (P = 0.002) (Table 2). Thus, based on the 56 total patients seen in the PDCC, each patient had a median savings of $7,424.40 ($3,335.30 - $16,725.50) (Table 3).
 Click to view | Table 2. Estimated Healthcare System Savings (Full Oral Feeds) |
 Click to view | Table 3. Estimated Healthcare System Savings (PDCC Group) |
Following discharge, for patients seen at the PDCC, 30-day ED revisit rates were 28.1% and 30-day readmissions were 22.8%, which were comparable to the control group rates of 27.4% 19.4%, respectively. Neither the ED revisit rates nor the readmission rates were significantly different between the groups (P = 0.937 and P = 0.644, respectively) (Table 4).
 Click to view | Table 4. PDCC Clinical Outcomes |
| Discussion | ▴Top |
This study emphasizes the utility in using a pediatric hospitalist-run PDCC. Several factors are suspected to contribute to the PDCC’s impact on decreased inpatient LOS. One of the most important is the employment of a full-time RN-case manager. This case-manager, who works under three physicians, is proficient in educating patients and families on NGT/NJT management both during, before, and after their PDCC appointment. The case-manager engages in follow-up calls with families after their appointments, coordinates 48-h post-discharge follow-up appointments, does medication reconciliation, and helps with triaging information. Therefore, this individual is vital in ensuring proper familial communication while addressing and triaging medical complexities.
The PDCC was subsequently able to follow an increased volume of discharged children with NGT/NJT, regardless of PCP or insurance status. The additional benefits of reduced LOS and associated cost savings secondary to PDCC utilization are also attributed to RN-case manager contributions. The resultant savings of approximately $954.38 per patient per day (range $418.21 - $2,199.30) equates to a cost reduction of about $100,000 per year ($954.38 × 26 × 4 days) over 2 years. Successful post-acute care is contingent on how post-discharge adverse events are anticipated and managed. It is expected that patients discharged with NGT/NJT will have questions regarding tube management between discharge and their scheduled outpatient visits. Both literature and our PDCC’s function support that RN-case managers have the knowledge base to troubleshoot these issues to avoid ED visits or readmissions through in-person PDCC visits or patient portal communications [12, 13]. Additional studies without PDCC follow-up have found comparable ED revisit rates to the control (26.4%) yet increased readmission rates (30.9%), though this was not significant [8]. In addition to being staffed by RN-case managers, having the PDCC staffed by hospitalists with ready access to patient charts can reduce caregiver and patient anxiety in comparison to being seen by PCPs with limited access to such records.
This pilot study favorably compares to cited readmission rates and ED visits in the current literature, with low tube-related readmission rates for NICU patients of 11% or 49% in general pediatric patients [8, 10]. These results support that pediatric patients can be safely discharged from inpatient services with nasoenteral feeding tubes [10-13]. This knowledge can be used to broaden the conditions, complexity, and ages of patients supported by a PDCC. This will further amplify the benefits that this clinic has on LOS and hospital throughput.
While our model may not have outperformed traditional post-discharge care models, such as those utilized by neonatologists, it did not underperform. We believe that this model demonstrates non-inferiority to long established care delivery models, such as the Home Enteral Feeding Transitions (HEFT) program model, that are current standards of practice [10]. Additionally, while the benefits of PDCCs on non-NICU populations remain understudied, they have shown promise in reducing ED visits and readmissions for our patient population [14]. Similar PDCC models have also effectively addressed insurance, transportation, and housing difficulties. More specifically, multiple PDCCs addressed the needs of low-income patients, showing decreased hospital revisits at 180 days post-discharge [15, 16]. Future studies should address the need for pediatric-specific PDCCs managed by hospitalists through cost-benefit analyses and the incorporation of longitudinal studies to track cost benefits over time.
This study underscores the value of the PDCC in offering alternative management strategies for post-discharge care in pediatric populations, particularly for those with NGT or NJT. This is relevant since in 2016, Pediatric Hospital Medicine was recognized as a subspecialty by the American Board of Pediatrics. Except for the Pediatric Critical Care Medicine, all other pediatric specialties have an outpatient footprint to follow patients discharged from the hospital. Thus, the PDCC bridges gaps in continuity of care by offering a promising approach for improved healthcare delivery while maintaining standards of care modeled by other pediatric subspecialties.
Furthermore, the PDCC model holds the potential for significant cost savings. Patients on tube feeds often do not need to be continuously monitored, but they remain admitted to the hospital. This leads to the consumption of pediatric beds, resources, and bed days, leading to increased hospital costs and throughput problems [17]. Our study reported a median savings of $24,813.80 per patient that achieved full oral feeds without increasing ED revisits or readmissions, equating to $211,000 - $322,000 saved per year through PDCC utilization. A comparable study at the University of Utah trialed a home enteral feeding transition program, which exhibited decreased LOS and increased cost savings for patients [10]. PDCCs represent source of incremental revenue and cost avoidance during a time when most hospitals are searching for ways to improve throughput and patient access in a fiscally responsible manner.
PDCCs play a crucial role in streamlining pediatric care post-discharge while simultaneously reducing spending. However, no standard process is in place for pediatric hospital discharges [18]. Children with medical complexities (such as NJT/NGT) often face adverse outcomes due to poor access to care and lack of care coordination [19]. Future directions at the national level should address additional need for and feasible ways to expand access to PDCCs. Eliciting PCP perspectives on managing complex pediatric patients after discharge, for example, may help identify the need for PDCCs. Telehealth PDCC visits could increase accessibility for families in rural communities [20]. Closer integration of clinics with hospitals and better alignment of financial incentives between them could further promote investment in and coordination with PDCCs. Additional studies should determine patient and caregiver satisfaction with utilizing PDCCs, as increased satisfaction may increase demand and desire for larger systems to support PDCC development.
Research has indicated that caregivers of children with medical complexities often experience heightened anxiety related to hospitalizations and the transition home [21]. Given the time and cost saving advantages of PDCCs, it is hypothesized that these services may also provide emotional benefits to families. By reducing stress and logistical burdens, PDCCs could create more opportunities for parents and children to bond during the critical post-discharge period. Future studies should explore how PDCC utilization may support pediatric neurodevelopment and strengthen caregiver-child bonding.
Strengths and limitations
The PDCC model utilized for pediatric patients with nasoenteric feeding tubes holds promise for various patient populations, some of which include those with mental health disorders (eating disorders), post-surgical care (wound management), and complex, chronic diseases (tracheostomies). The cost benefits observed from the PDCC are also applicable to patients without insurance or PCPs.
This study has several limitations. First, we only included patients less than 2 years old for analysis. This excludes a variety of pediatric conditions and ages where tube feeds are necessary secondary to the presence of chronic conditions, such as Chron’s disease, complex neurological conditions, or eating disorders. However, while this may have decreased external validity, this benefited our design as it allowed us to directly focus on patients who utilized short-term NGT/NJTs. Second, the small sample size from a single institution with specific protocols limits the generalizability of the results. However, using this PDCC as a pilot for other clinics requires an analysis of a discrete subpopulation of patients prior to broadly implementing the PDCC for additional conditions and ages. Further, the decrease in LOS and cost benefits were estimates made as part of this retrospective analysis.
Thirdly, the control group was a limitation of this study. Future studies should assess how a PDCC compares to PCP or subspecialty (ENT, GI, etc.) management of NJT/NGT by assessing readmission rates, ED visits, and LOS for children discharged home with NJT/NGT. However, considering this limitation, we believe that this study highlights the effectiveness of PDCC reducing costs and LOS for pediatric populations who would otherwise stay hospitalized until their NJT/NGT was removed based on previously established standards of care.
Another limitation is the expectation that cost distribution was constant across the patient’s entire LOS. More diagnostic tests and procedures are run earlier in hospital stays, leading to a potential underestimate of cost reduction benefits of PDCC use for analysis. Finally, cost savings did not account for inflation, once again potentially underestimating PDCC cost savings. However, we believe that the results found still stand in significance as the control data (2017 - 2019) ended up having greater expenditure than the PDCC (2019 - 2021) group, even in lieu of rising hospital costs with each passing year. Future research should incorporate a larger, more diverse patient population and add a prospective component for data analysis to more ideally assess the impact of the PDCC on various pediatric patient populations. One prospective point of interest could include focusing on outcomes in those who achieved full oral feeds compared to those who did not.
A final limitation of this study lies in the lack of control for certain demographic variables, such as pediatric weight, presence of chromosomal conditions contributing to dysphagia, and ICU admission rates, between the PDCC and control groups. Notably, ICU admissions were significantly higher in the control group, which may have contributed to the increased costs observed in that cohort. However, given the relatively small sample size, we chose not to control for these demographic variables, as doing so would have required the exclusion of additional cases and further limited the study’s power. Additionally, because there were no significant differences in ED visits or 30-day readmissions, we believe that ICU admission rates did not significantly impact the results [22]. Despite this limitation, we believe that the findings underscore the valuable role that PDCCs can play in enhancing pediatric patient care.
Conclusion
This study underscores the cost saving and medical advantages of PDCC utilization, particularly PDCCs staffed by RN-case managers and pediatric hospitalists. The most prominent benefit of this model includes decreased inpatient LOS. The PDCC likely serves to improve access to care while minimizing adverse patient outcomes. Despite its limitations, this study represents a proof of concept how the PDCC improves transition from inpatient to outpatient environments, particularly for patients discharged with NGT or NJT. This study also supports the potential for increased hospital capacity secondary to decreased patient LOS for PDCC patients. While these results are promising, additional research needs to be applied to more complex pediatric populations. The PDCC continues to show potential in decreasing healthcare spending and improving healthcare delivery, all while minimizing adverse patient outcomes, making prioritization of this model critical.
Acknowledgments
We would like to express our sincere appreciation to Bryce Evans, Penn State College of Medicine, as well as the RN-case managers at the Penn State Transitions of Care Clinic for their continued support throughout our study.
Financial Disclosure
This project was completed without financial support.
Conflict of Interest
The authors have no conflict of interest to disclose.
Informed Consent
Informed consent was not required, as this study involved a retrospective review of de-identified clinical data.
Author Contributions
Jessica Santucci aided in conceptualization of the study, collected the study data, authored the initial manuscript, and assisted with manuscript revisions. Meghan Hartman aided in the collection of data and assisted with manuscript authorship and revisions. Dr. Nelson conceptualized the study, oversaw data collection, and assisted with manuscript revisions. Dr. Beck conceptualized the study and assisted with manuscript revisions. Dr. King ran statistical analyses on the collected data, aided in interpretation of the data, and assisted with manuscript writing and revisions. All authors approve of the submitted manuscript and take ownership of the resultant work.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
ED: emergency department; G-tube: gastrostomy tube; ICD-10: International Classification of Diseases, 10th Revision; IQR: interquartile range; LOS: length of stay; NGT/NJT: nasogastric/nasojejunal tube; PCP: primary care physician; PDCC: post-discharge care clinic; RN-case manager: registered nurse-case manager; SAS: Statistical Analysis System
| References | ▴Top |
- Beck MJ, Gosik K. Redesigning an inpatient pediatric service using Lean to improve throughput efficiency. J Hosp Med. 2015;10(4):220-227.
doi pubmed - Burke RE, Ryan P. Postdischarge clinics: hospitalist attitudes and experiences. J Hosp Med. 2013;8(10):578-581.
doi pubmed - Burke RE, Whitfield E, Prochazka AV. Effect of a hospitalist-run postdischarge clinic on outcomes. J Hosp Med. 2014;9(1):7-12.
doi pubmed - Doctoroff L. Interval examination: establishment of a hospitalist-staffed discharge clinic. J Gen Intern Med. 2012;27(10):1377-1382.
doi pubmed - Doctoroff L, Nijhawan A, McNally D, Vanka A, Yu R, Mukamal KJ. The characteristics and impact of a hospitalist-staffed post-discharge clinic. Am J Med. 2013;126(11):1016.e1019-1015.
doi pubmed - Doctoroff L. Postdischarge clinics and hospitalists: a review of the evidence and existing models. J Hosp Med. 2017;12(6):467-471.
doi pubmed - Kuo S, Aledia A, O'Connell R, Rudkin S, Dangodara AA, Amin AN. Implementation and impact on length of stay of a post-discharge remote patient monitoring program for acutely hospitalized COVID-19 pneumonia patients. JAMIA Open. 2022;5(3):ooac060.
doi pubmed - Rosen D, Schneider R, Bao R, Burke P, Ceballos C, Hoffstadter-Thal K, Benkov K. Home nasogastric feeds: feeding status and growth outcomes in a pediatric population. JPEN J Parenter Enteral Nutr. 2016;40(3):350-354.
doi pubmed - White BR, Ermarth A, Thomas D, Arguinchona O, Presson AP, Ling CY. Creation of a standard model for tube feeding at neonatal intensive care unit discharge. JPEN J Parenter Enteral Nutr. 2020;44(3):491-499.
doi pubmed - White BR, Zhang C, Presson AP, Friddle K, DiGeronimo R. Prevalence and outcomes for assisted home feeding in medically complex neonates. J Pediatr Surg. 2019;54(3):465-470.
doi pubmed - Williams SL, Popowics NM, Tadesse DG, Poindexter BB, Merhar SL. Tube feeding outcomes of infants in a Level IV NICU. J Perinatol. 2019;39(10):1406-1410.
doi pubmed - Smith J, Taylor M. Managing feeding tube complications: the role of parental education. J Pediatr. 2021;235:123-130.
doi - Ahearn MA, Stephens JR, Zwemer EK, Hall M, Ahuja A, Chatterjee A, Coletti H, et al. Characteristics and outcomes of children discharged with nasoenteral feeding tubes. Hosp Pediatr. 2022;12(11):969-980.
doi pubmed - Rotenstein L, Melia C, Samal L, Pollack S, Yu N, Cunningham R, Price C. Development of a primary care transitions clinic in an academic medical center. J Gen Intern Med. 2022;37(3):582-589.
doi pubmed - Liss DT, Ackermann RT, Cooper A, Finch EA, Hurt C, Lancki N, Rogers A, et al. Effects of a transitional care practice for a vulnerable population: a pragmatic, randomized comparative effectiveness trial. J Gen Intern Med. 2019;34(9):1758-1765.
doi pubmed - Kangovi S, Mitra N, Grande D, White ML, McCollum S, Sellman J, Shannon RP, et al. Patient-centered community health worker intervention to improve posthospital outcomes: a randomized clinical trial. JAMA Intern Med. 2014;174(4):535-543.
doi pubmed - Makker J, Pardy C, Kelly V, Yardley I. The community cost of maintaining gastrostomies in pediatric patients. JPGN Rep. 2023;4(1):e278.
doi pubmed - Berry JG, Blaine K, Rogers J, McBride S, Schor E, Birmingham J, Schuster MA, et al. A framework of pediatric hospital discharge care informed by legislation, research, and practice. JAMA Pediatr. 2014;168(10):955-962.
doi pubmed - Glick AF, Farkas JS, Magro J, Shah AV, Taye M, Zavodovsky V, Rodriguez RH, et al. Management of discharge instructions for children with medical complexity: a systematic review. Pediatrics. 2023;152(5):e2023061572.
doi pubmed - Nelson A, Stuckey H, Snyder B, Van Scoy LJ, Daymont C, Irvin C, Wasserman E, et al. Provider perspectives of transitions of care at a tertiary care children's hospital with a hospitalist-run discharge clinic. Clin Pediatr (Phila). 2023;62(8):926-934.
doi pubmed - Mackay L, Dewan T, Lal J, Hayden KA, Chang U. Exploration of the evidence on discharge from hospital to home for children with medical complexity and their parents: a review of the literature. Child Care Health Dev. 2025;51(1):e70031.
doi pubmed - Hirshberg EL, Wilson EL, Stanfield V, Kuttler KG, Majercik S, Beesley SJ, Orme J, et al. Impact of critical illness on resource utilization: a comparison of use in the year before and after ICU admission. Crit Care Med. 2019;47(11):1497-1504.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
International Journal of Clinical Pediatrics is published by Elmer Press Inc.