| International Journal of Clinical Pediatrics, ISSN 1927-1255 print, 1927-1263 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Int J Clin Pediatr and Elmer Press Inc |
| Journal website https://ijcp.elmerpub.com |
Original Article
Volume 14, Number 2, October 2025, pages 37-43
Preliminary Experience With a Lidocaine Infusion as an Adjunct During Acute Pain Management Related to Medical Illnesses in Pediatric Patients
Edison E. Villalobosa, Sibelle Aurelie Yemele Kitioa, Catherine Rotha, Maria Shepardb, c, Ambrish Patela, d, Joseph D. Tobiasa, d, e
aDepartment of Anesthesiology and Pain Medicine, Nationwide Children’s Hospital, Columbus, OH, USA
bHeritage College of Osteopathic Medicine - Cleveland Campus, Warrensville Heights, OH, USA
cOhio University, Athens, OH, USA
dDepartment of Anesthesiology and Pain Medicine, The Ohio State University College of Medicine, Columbus, OH, USA
eCorresponding Author: Joseph D. Tobias, Department of Anesthesiology and Pain Medicine, Nationwide Children’s Hospital, Columbus, OH 43205, USA
Manuscript submitted August 29, 2025, accepted September 24, 2025, published online October 17, 2025
Short title: Lidocaine Infusion and Analgesia
doi: https://doi.org/10.14740/ijcp1024
| Abstract | ▴Top |
Background: The potential analgesic effects of lidocaine have been reported in anecdotal reports and larger clinical trials in adults. However, there remains limited information regarding its use in pediatric-aged patients. In many institutions, continuous lidocaine infusions may be restricted to the pediatric intensive care unit (ICU) setting. This study assessed the safety and feasibility of the use of lidocaine outside the ICU setting as an adjunct for acute pain management related to medical conditions in pediatric patients.
Methods: We retrospectively reviewed the outcomes of patients ≤ 21 years of age with acute medical illnesses, who received a continuous lidocaine infusion on the inpatient ward for the management of acute pain related to medical illnesses.
Results: The study cohort included 15 patients who received a total of 46 lidocaine infusions during the study period. Patient ages ranged from 10 to 21 years (median age: 14 years, interquartile range (IQR): 13 - 20 years) with a median weight of 57.9 kg (IQR: 54 - 74 kg). The majority of encounters (n = 43, 94%) were for pain related to sickle cell disease. Most encounters (n = 31; 67%) received continuous lidocaine infusions for 1 - 7 days at a median dose of 1 mg/kg/h. Adverse events were noted on 11 of the 310 infusion days (4%), and at least one adverse event was reported in nine of the 46 encounters (20%). Infusion rate adjustments were made in six encounters (13%) due to adverse events. No adverse effects required escalation of the level of care.
Conclusions: This study, which outlines our use of lidocaine as an adjunct to pain management for acute pain related to acute medical illnesses, expands on our previous work which described lidocaine for surgical pain. The study provides evidence toward the safety of pre-developed pathways and protocols for continuous lidocaine infusions outside the ICU setting, making lidocaine a feasible, non-opioid adjunct for the treatment of acute pain related to medical illnesses.
Keywords: Lidocaine infusion; Multimodal analgesia; Acute pain; Postoperative analgesia; Sickle cell disease
| Introduction | ▴Top |
Lidocaine is a local anesthetic of the amide class. It was initially synthesized in the 1940s and released for clinical use in 1948, initially as a local anesthetic agent [1, 2]. Since its release for clinical use, it has been used for the treatment of arrhythmias, neuraxial anesthesia, and superficial infiltration for cutaneous analgesia. Anecdotal experience shortly after its release for clinical use also suggested its potential analgesic effects [2]. Several prospective trials in adults have demonstrated beneficial acute and long-term perioperative effects on acute post-surgical pain with improvements in pain scores, decreased opioid needs, anti-hyperalgesic actions, and anti-inflammatory effects [2]. However, there remains limited information regarding its use in pediatric-aged patients for the treatment of acute pain. Additionally, in many institutions, continuous lidocaine infusions are restricted to the pediatric intensive care unit (PICU) setting due to concerns regarding its adverse effect profile and the need for monitoring. We present our initial retrospective experience regarding the safety profile of lidocaine infusions on the inpatient ward as an adjunct for pain management in pediatric patients with acute pain related to medical illnesses. We also describe the development of our protocol and pathway involved in the use of this therapy outside the PICU setting.
| Materials and Methods | ▴Top |
This retrospective study was approved by the Institutional Review Board of Nationwide Children’s Hospital. As this was a retrospective study using deidentified data, the need for individual written informed consent was waived. The study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Study cohort and data acquisition
The study cohort included patients ≤ 21 years of age who received a lidocaine infusion for the management of acute pain related to a medical illness over an 18-month period, following the development of a pathway and an institutional policy for the administration of lidocaine infusions as an adjunct to pain management on the inpatient ward, outside the PICU setting. Information regarding the development of this protocol and its requirements is outlined below. Patients receiving a therapeutic lidocaine infusion for control of arrhythmias or other therapeutic indications were excluded.
Patients were identified, and a patient list was created using records from the Acute Pain Service, pharmacy database, and the EPIC Data Warehouse. For each patient, demographic data collected included age, gender, weight, primary disease process or pain condition, and comorbid conditions. Information collected regarding the lidocaine infusion included the infusion rate (mg/kg/h), adjustments to the infusion rate including the new infusion rate, reasons if the lidocaine infusion was stopped, and total duration (days) of lidocaine therapy. Adverse events were also recorded each day for patients while on the lidocaine infusions, including the need to stop the lidocaine infusion or change the infusion rate. End-organ function was assessed by reviewing all laboratory data, including but not limited to complete blood count, renal function, and hepatic function. Baseline and subsequent electrocardiogram (ECG) data were reviewed. Lidocaine levels were not routinely obtained as part of the protocol (see below). Escalations of the level of care (from the inpatient ward to the PICU) or the need to notify the rapid response team were identified.
Data presentation and statistical analysis
Data were collected through research electronic data capture (REDCap) and analyzed using Stata software (version 18; StataCorp, College Station, TX). Descriptive statistics were the primary focus of the analysis. For continuous variables, medians and interquartile ranges (IQRs) were reported. Categorical variables were summarized with counts and percentages.
Lidocaine protocol
Prior to the start of this initiative, a lidocaine policy and protocol was created for the hospital, which outlined the use of a lidocaine infusion on the inpatient ward as an adjunct to opioids for pain management. This pathway has been previously reported as it pertains to our use of continuous lidocaine infusions for the treatment of acute surgical pain [3]. Through the hospital’s online learning system, educational modules were provided to the inpatient nursing staff who would be charged with caring for these patients. This education provided information about the use of lidocaine for analgesia, dosing, adverse effects, patient monitoring, and documentation requirements in the electronic medical record (EMR). After completion of the module, the nurses completed a test to receive credit for completing the module. Information regarding the use of lidocaine for analgesia was also added to the hospital’s pain management intranet website. The use of lidocaine infusions started on the inpatient ward for Hematology and Oncology patients in March 2020 and was subsequently expanded to other inpatient floors.
Lidocaine infusions for analgesia in patients on the inpatient wards were managed through consultation with either the Acute Pain Service or the Palliative Care Service. A baseline ECG was obtained if one had not been obtained within 30 days of the infusion. If no arrhythmias of significance were noted, ongoing continuous ECG monitoring is not required during the infusion on the inpatient ward. Other monitoring modalities included continuous oxygen saturation using pulse oximetry. Blood pressure, heart rate, respiratory rate, sedation, and level of consciousness were monitored at initiation of the infusion, every 30 min for 1 h, hourly for 2 h, and then every 4 h during the remainder of the infusion. The presence of symptoms of central nervous system toxicity (tinnitus, blurred vision, confusion, altered mental status, slurred speech) was documented every 30 min for the first hour, and then every hour during the infusion. Pain scores were monitored per the hospital protocol.
An order set was created in the EMR system to include the necessary dosing and monitoring information for the lidocaine infusion for analgesia protocol. Dosing for acute pain management included a lidocaine infusion initiated at 1 mg/kg/h. A bolus dose of 1 mg/kg was optional prior to starting the infusion. For obese patients, ideal body weight was used for dosing. The infusion rate was increased as needed to a maximum rate of 3 mg/kg/h or 200 mg/h.
| Results | ▴Top |
The study cohort included 15 patients who received a total of 46 lidocaine infusions during the study period. Patient ages ranged from 10 to 21 years (median age 14 years, IQR: 13 - 20 years). The median weight was 57.9 kg (IQR: 54.1 - 74.4 kg), and the median height was 160.7 cm (IQR: 151.9 - 169.8 cm). Demographic and clinical characteristics, including primary disease, indication for infusion, and hospital length of stay, are summarized for each encounter in Table 1. The majority of encounters (n = 43, 94%) were for pain related to sickle cell disease. The median hospital length of stay was 7 days (IQR: 5 - 12 days).
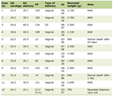 Click to view | Table 1. Demographic and Clinical Characteristics |
Lidocaine was infused for 1 to 16 days per encounter, for a total of 310 infusion days across the 46 encounters. The infusion dose ranged from 0.5 to 2.5 mg/kg/h. In 31 encounters (67%), continuous lidocaine infusions were administered for 1 to 7 days at a median dose of 1 mg/kg/h. At least one lidocaine bolus was administered in 41% of encounters (n = 19), with a total of 20 boluses administered. The median bolus dose was 2.0 mg/kg (IQR: 1.2 - 2.0 mg/kg). Infusion characteristics are summarized in Table 2.
 Click to view | Table 2. Lidocaine Infusion Characteristics |
Adverse events were noted on 11 of the 310 infusion days (4%). Nine of the 46 encounters (20%) were associated with adverse events, including hypotension (n = 4), dizziness (n = 3), anxiety (n = 1), and hallucinations (n = 1). Eight were associated with a single adverse event (17%), and one with three episodes of hypotension. Infusion rate adjustments were made in six encounters (13%) due to adverse events, including hypotension (n = 3), dizziness (n = 2), and hallucination (n = 1). Adverse events details are shown in Table 3.
 Click to view | Table 3. Adverse Events During Lidocaine Infusion |
| Discussion | ▴Top |
The current study expands on our previous work describing our use of lidocaine for acute pain following surgical procedures [3]. In our previous study, 168 patients were retrospectively reviewed; of that cohort, a total of 148 patients (88%) received a lidocaine infusion initiated intraoperatively, and 31 (19%) of these patients received a bolus dose prior to the infusion. The majority of patients (86%) were maintained on a continuous lidocaine infusion for 1 - 3 postoperative days at an average dose of 1 mg/kg/h, with only four patients receiving the infusions beyond 7 days. Adverse events were noted in 29 patients (17%), including dizziness, hallucinations, anxiety, and hypotension. A similar adverse event profile and rate (20%) was noted in the current study. Although the same infusion protocol was used, its application in a different clinical context, adjunct to pain management for acute pain related to medical illnesses, allowed for observation of infusions extending up to 16 days. This provides additional evidence toward the safety and feasibility of continuous lidocaine infusion, as it may be tolerated over extended periods of time. Additionally, we report our process and pathway for the use of lidocaine infusions in pediatric-aged patients outside an ICU setting.
Anecdotal experience with lidocaine to treat acute pain in pediatric patients has been reported to be effective [4]. Nathan et al described an 11-year-old male with erythromelalgia, in whom all pain management techniques had failed, experiencing approximately 20 episodes of acute pain per day. The initiation of intravenous (IV) lidocaine at 1 mg/kg/h, titrated to maintain a therapeutic serum level of 2 - 5 µg/mL, reduced the number of pain episodes to 1 per day. Pain management was later transitioned from IV lidocaine to oral mexiletine, a class 1B antiarrhythmic analog of lidocaine, improving symptoms and allowing return to normal activities. Additional clinical work has suggested the potential efficacy of IV lidocaine as an adjunct to pain of various etiologies including perioperative pain, cancer pain, complex regional pain syndrome, and different forms of neuropathic pain, where conventional therapies have failed, including erythromelalgia, diabetic neuropathy, and sickle cell disease [4-8]. Notably, 94% of patients in our cohort had acute pain related to sickle cell disease as their primary condition.
The presence of neuropathic pain is often associated with injury to neurons in the central and peripheral nervous system, leading to abnormal sodium channel activity. Lidocaine, by blocking voltage-gated sodium channels, may reduce neuronal hyperexcitability and improve symptoms. Although this is believed to be the primary mechanism responsible for its antinociceptive effect, lidocaine also interacts with other ion channels, receptors, and molecular targets, suggesting a complex set of pathways. Moreover, findings indicate that its clinical effects may persist for several days, even after plasma levels decline significantly. Further research is needed to better define these mechanisms and explore potential opportunities in pain therapy [1, 9].
Despite the potential benefits, the safety and feasibility of lidocaine infusions have been an ongoing debate due to the known adverse events profile, including cardiovascular effects such as arrhythmias, hypotension, and cardiac arrest, central nervous system effects leading to confusion, dizziness, sedation, seizures, ataxia, and respiratory effects such as shortness of breath. Given these concerns, the ICU is often considered the optimal setting for lidocaine infusion therapy, offering continuous telemetry, frequent vital signs monitoring, and immediate access to advanced cardiovascular life support, with a clear ability to monitor for signs of overdose or toxicity [4, 10-12]. The perioperative setting also offers similar advantages and has been used as a setting to provide continuous and close monitoring, demonstrating the safety of short-term lidocaine infusions for the control of acute surgical pain [3, 12, 13]. However, experience with lidocaine infusions outside the ICU or perioperative areas is limited. With proper preparation including standardized protocols and properly trained staff, the current cohort provides additional data for the safety of lidocaine infusions outside the ICU setting. Additional information supporting the safe use of lidocaine infusions for acute pain management is summarized in Table 4 [3, 4, 10-18]. In specific instances, the lidocaine infusion was initiated in the ICU or operating room and continued on the inpatient ward. A similar protocol applied to a larger cohort of patients with acute pain related to sickle cell vaso-occlusive crisis has been described [18]. Similar to our work, the authors outline their institutional protocol regarding the safe use of lidocaine infusions outside the ICU setting. It should also be noted that the study of Luo et al, which was a prospective, randomized, double-blinded trial in a cohort of 38 adolescents (10 - 19 years of age) undergoing single state scoliosis surgery showed no postoperative analgesic benefit (morphine requirements and pain scores) with the administration of lidocaine starting intraoperatively and continued for a total of 48 h [17].
 Click to view | Table 4. Summary of Pediatric Reports Using Lidocaine Infusions |
Lidocaine serum levels were not routinely obtained as part of our clinical practice. End-organ function, drug interactions, and associated comorbid conditions may affect serum concentrations of lidocaine, particularly in pediatric-aged patients [1, 2, 19]. As a result, our Acute Pain Service developed individualized pain plans, where initial dosing and subsequent adjustments were performed according to ongoing assessment of the therapeutic response of the patients. Although adverse effects were reported in nine of the 46 encounters (20%), a single patient with significant comorbidities experienced four of these events. This patient, diagnosed with sickle cell disease, functional asplenia, and opioid use disorder, was treated for vaso-occlusive crisis multiple times, with a regimen including lidocaine infusions, ibuprofen, and tizanidine in addition to opioids. The patient experienced hypotension (n = 3) and hallucinations (n = 1). As a result, the patient was later switched from tizanidine to cyclobenzaprine to eliminate the impact of tizanidine on blood pressure, a well described adverse effect of this centrally acting α2-adrenergic agonist [20]. This case highlights the complexity of managing multimodal analgesia with lidocaine infusions when used with other pharmacologic agents. In the remaining five encounters, adverse events were managed by dose adjustments or discontinuation of the infusion, without further concerns or the need to add additional pharmacologic agents to address the adverse effect. The low incidence of adverse effect and its limited impact on physiologic well-being was significant, especially when caring for a cohort that included medically complex patients, many with underlying conditions including sickle cell disease, functional asplenia, acute chest syndrome, opioid use disorder, immunocompromise, asthma, and fever. Although a direct analysis of pain outcomes was not performed, a subjective analysis by clinicians caring for these patients demonstrates that the majority of patients experienced improved pain control and decreased opioid requirements during the study period. This is consistent with similar trends described in previous reports (Table 4) [3, 4, 10-18].
Specific limitations of the current study should be recognized. In addition to the retrospective nature of the study and the small sample size, including patients (n = 15) and encounters (n = 46), the beneficial and adverse effects of lidocaine can be masked due to the co-administration of other analgesic or sedative agents with overlapping pharmacologic profiles. The majority of patients studied had acute pain related to sickle cell crisis, thereby limiting our ability to comment on the efficacy of lidocaine in acute pain related to other medical illnesses. In general, our patient cohort was older (age range 10 - 21 years with a median age of 14 years). As such, applicability to younger patients may not be feasible, and additional data regarding safety are needed. Without a control group, the direct efficacy of lidocaine or the causality of its adverse effects cannot be determined. Pain score improvement may be due to multimodal analgesia, not lidocaine alone. The decision to use a lidocaine infusion, when it was started, when it was discontinued, and the use of adjunctive medications was at the discretion of the attending on the acute pain service. We recognize that co-administered medications can be responsible for clinical changes that require adjusting the dose or stopping the infusion, affecting how feasible lidocaine truly is in this setting. This manuscript does not claim causality or efficacy of lidocaine; instead, it focuses on safety, feasibility outside the ICU, and tolerance in pediatric patients by reviewing infusion data and adverse effect profile.
In summary, we present our preliminary experience with the administration of a lidocaine infusion outside the ICU setting as an adjunct for acute pain management in pediatric patients with medical illnesses. The study cohort included 15 patients, 46 encounters, and a total of 310 infusion days, supporting feasibility over time in the inpatient ward setting. Most infusions were well tolerated, and no life-threatening adverse events were found. Overall, our findings suggest that a standardized lidocaine infusion protocol managed by a specialized interdisciplinary team may offer a safe and feasible alternative for pain management, particularly in cases where conventional analgesic therapies have failed. Duplication of our process with the use of lidocaine infusions outside the ICU setting requires that the institution is well equipped and served by multi-disciplinary teams to manage such care. Future studies are needed to further clarify the role of lidocaine infusions in the management of acute pain related to surgical and medical etiologies.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
As this was a retrospective study using deidentified data, the need for individual written informed consent was waived.
Author Contributions
Preparation of initial, subsequent, and final drafts: EEV; data collection, review of drafts including final document: CR, MS, AP; data analysis, review of drafts including final version: SAYK; concept, writing, and review of all drafts: JDT.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
| References | ▴Top |
- Hermanns H, Hollmann MW, Stevens MF, Lirk P, Brandenburger T, Piegeler T, Werdehausen R. Molecular mechanisms of action of systemic lidocaine in acute and chronic pain: a narrative review. Br J Anaesth. 2019;123(3):335-349.
doi pubmed - Dunn LK, Durieux ME. Perioperative use of intravenous lidocaine. Anesthesiology. 2017;126(4):729-737.
doi pubmed - Couser DF, Roth C, Holbrook M, Yemele Kitio SA, Wrona S, Martin D, et al. Preliminary experience with a continuous lidocaine infusion as an analgesia adjunct for acute pain management following surgery in pediatric patients. Int J Clin Pediatr. 2023;12(2):29-36.
- Nathan A, Rose JB, Guite JW, Hehir D, Milovcich K. Primary erythromelalgia in a child responding to intravenous lidocaine and oral mexiletine treatment. Pediatrics. 2005;115(4):e504-507.
doi pubmed - Bach FW, Jensen TS, Kastrup J, Stigsby B, Dejgard A. The effect of intravenous lidocaine on nociceptive processing in diabetic neuropathy. Pain. 1990;40(1):29-34.
doi pubmed - Viola V, Newnham HH, Simpson RW. Treatment of intractable painful diabetic neuropathy with intravenous lignocaine. J Diabetes Complications. 2006;20(1):34-39.
doi pubmed - Brandow AM, Farley RA, Panepinto JA. Neuropathic pain in patients with sickle cell disease. Pediatr Blood Cancer. 2014;61(3):512-517.
doi pubmed - Nguyen NL, Kome AM, Lowe DK, Coyne P, Hawks KG. Intravenous lidocaine as an adjuvant for pain associated with sickle cell disease. J Pain Palliat Care Pharmacother. 2015;29(4):359-364.
doi pubmed - Lai J, Porreca F, Hunter JC, Gold MS. Voltage-gated sodium channels and hyperalgesia. Annu Rev Pharmacol Toxicol. 2004;44:371-397.
doi pubmed - Mooney JJ, Pagel PS, Kundu A. Safety, tolerability, and short-term efficacy of intravenous lidocaine infusions for the treatment of chronic pain in adolescents and young adults: a preliminary report. Pain Med. 2014;15(5):820-825.
doi pubmed - Lo C, Le S, Bell M, Kim E. Continuous intravenous lidocaine provides effective pain control in a palliative child: a case report. A A Pract. 2020;14(11):e01305.
doi pubmed - Lemming K, Fang G, Buck ML. Safety and tolerability of lidocaine infusions as a component of multimodal postoperative analgesia in children. J Pediatr Pharmacol Ther. 2019;24(1):34-38.
doi pubmed - Gupta A, Ashok V. Perioperative intravenous lignocaine for pediatric postoperative pain-A systematic review and meta-analysis. Paediatr Anaesth. 2025;35(1):25-32.
doi pubmed - Wallace MS, Lee J, Sorkin L, Dunn JS, Yaksh T, Yu A. Intravenous lidocaine: effects on controlling pain after anti-GD2 antibody therapy in children with neuroblastoma—a report of a series. Anesth Analg. 1997;85(4):794-796.
doi pubmed - Massey GV, Pedigo S, Dunn NL, Grossman NJ, Russell EC. Continuous lidocaine infusion for the relief of refractory malignant pain in a terminally ill pediatric cancer patient. J Pediatr Hematol Oncol. 2002;24(7):566-568.
doi pubmed - Gibbons K, DeMonbrun A, Beckman EJ, Keefer P, Wagner D, Stewart M, Saul D, et al. Continuous lidocaine infusions to manage opioid-refractory pain in a series of cancer patients in a pediatric hospital. Pediatr Blood Cancer. 2016;63(7):1168-1174.
doi pubmed - Luo J, West N, Pang S, Golam A, Adams E, Gorges M, Carr RR, et al. Perioperative intravenous lidocaine infusion therapy as an adjunct to multimodal analgesia for adolescent idiopathic scoliosis surgical correction: a double-blind randomized controlled trial. Paediatr Anaesth. 2025;35(7):552-561.
doi pubmed - Agbakwuru U, AuBuchon JD, Toebe B, LaBarge A, Di Paola J, Hulbert ML. Safety and tolerability of intravenous lidocaine infusions as opioid adjunct for children hospitalized with sickle cell Vaso-occlusive pain. Pediatr Blood Cancer. 2025;72(3):e31458.
doi pubmed - Batchelor HK, Marriott JF. Paediatric pharmacokinetics: key considerations. Br J Clin Pharmacol. 2015;79(3):395-404.
doi pubmed - Wagstaff AJ, Bryson HM. Tizanidine. A review of its pharmacology, clinical efficacy and tolerability in the management of spasticity associated with cerebral and spinal disorders. Drugs. 1997;53(3):435-452.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
International Journal of Clinical Pediatrics is published by Elmer Press Inc.