| International Journal of Clinical Pediatrics, ISSN 1927-1255 print, 1927-1263 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Int J Clin Pediatr and Elmer Press Inc |
| Journal website https://ijcp.elmerpub.com |
Original Article
Volume 000, Number 000, October 2025, pages 000-000
A Retrospective Review to Determine the Safety of Continuous Dexmedetomidine Infusions on the Inpatient Ward in Pediatric Patients With Acute and Chronic Pain Conditions
Aubrey Wronaa, b, Sibelle Aurelie Yemele Kitioc, Catherine Rothc, Sharon Wronac, Joseph D. Tobiasc, d, e
aHeritage College of Osteopathic Medicine - Dublin Campus, Dublin, OH, USA
bOhio University, Athens, OH, USA
cDepartment of Anesthesiology and Pain Medicine, Nationwide Children’s Hospital, Columbus, OH, USA
dDepartment of Anesthesiology and Pain Medicine, The Ohio State University College of Medicine, Columbus, OH, USA
eCorresponding Author: Joseph D. Tobias, Department of Anesthesiology and Pain Medicine, Nationwide Children’s Hospital, Columbus, OH 43205, USA
Manuscript submitted May 30, 2025, accepted July 24, 2025, published online October 10, 2025
Short title: Dexmedetomidine Use in Pediatric Inpatients
doi: https://doi.org/10.14740/ijcp1010
| Abstract | ▴Top |
Background: Dexmedetomidine is an α2-adrenergic receptor agonist that has clinical efficacy in various clinical scenarios in infants and children including procedural sedation, prevention of emergence delirium, and sedation during mechanical ventilation. Additional investigation has suggested its utility in various acute pain, chronic pain, and palliative care scenarios. Despite these beneficial properties, many pediatric institutions limit its use to the pediatric intensive care unit (ICU) setting. We present the development of a hospital-based pathway for the administration of dexmedetomidine on the inpatient ward. Additionally, we present preliminary dosing parameters and safety data using dexmedetomidine along this clinical pathway.
Methods: This retrospective chart review included patients ≤ 21 years of age who received a dexmedetomidine infusion for the management of acute or chronic pain outside the ICU setting. In addition to demographic data, information collected included the patient’s primary disease process, primary pain etiology, and comorbid conditions. Information to identify adverse effects included the need to stop the infusion, administration of vasoactive medications, provision of airway or respiratory support, or escalation of care with ICU admission or notification of the rapid response team.
Results: The study cohort included 26 patients (median age 5 years). The majority of patients (46%) received dexmedetomidine for 1 or 2 days. The most common primary disease conditions were neuroblastoma (n = 12), acute leukemia (n = 6), and sickle cell disease (n = 4). The starting infusion rate was generally 0.1 µg/kg/h, titrated up in 0.05 µg/kg/h increments to a maximum infusion rate of 0.6 µg/kg/h. For hospice patients with an allow natural death (AND) order, the maximum infusion rate was 1 µg/kg/h. Adverse effects (AEs) were reported in 11 patients, with the most common being hypotension (n = 4) and delirium or hallucinations (n = 4). No pharmacologic intervention was required due to AEs.
Conclusions: Our preliminary experience supports the safe use of dexmedetomidine infusions outside the ICU setting following a standardized protocol that includes patient selection, dosing parameters, monitoring, and training of physicians and nurses.
Keywords: Dexmedetomidine; Pain management; Sedation; Adverse effect
| Introduction | ▴Top |
Dexmedetomidine, like clonidine, is an α2-adrenergic receptor agonist that was approved by the United States Food and Drug Administration (FDA) in 1999 for the short-term (less than 24 h) sedation for adult intensive care unit (ICU) patients, who were receiving mechanical ventilation with endotracheal intubation [1, 2]. Subsequently, dexmedetomidine also received FDA-approval for sedation in the operating room setting or monitored anesthesia care (MAC) in adults. The physiological effects of dexmedetomidine are mediated through the α2-adrenergic receptor inducing the downregulation of intracellular cyclic adenosine monophosphate (AMP) [3]. Cyclic AMP downregulation causes alterations in ion channels, ion translocation, and membrane conductance. These changes decrease neuronal activation resulting in the clinical effects of sedation and anxiolysis [4]. Other physiological effects of dexmedetomidine include decreased heart rate (HR) and blood pressure (BP) through centrally acting α2-adrenergic agonism, which reduces central norepinephrine output, resulting in sympatholytic effects. Decreased noradrenergic output from the locus coeruleus induces increased firing of y-amino butyric acid and other inhibitory neurons [5]. Additional analgesic effects result from decreased release of substance P within the dorsal horn of the spinal cord.
The combination of dexmedetomidine’s favorable sedative effects with a limited adverse effect profile have led to its increased use in the pediatric population in various clinical settings including procedural sedation, prevention of emergence delirium following general anesthesia, sedation during mechanical ventilation, and intraoperative administration to supplement general anesthesia. Anecdotal clinical experience has also demonstrated dexmedetomidine’s role in acute pain management, chronic pain control, as well as palliative and hospice care in pediatric patients [6]. Reported benefits in these clinical scenarios have included improved analgesia and anxiolysis, a reduction in dose requirements for other sedative and analgesic agents, control of delirium, and minimizing the adverse effects of other medications. Despite its favorable safety profile and high therapeutic index, the administration of dexmedetomidine infusions has generally been limited to the pediatric intensive care unit (PICU) setting. We present the development of our hospital-based pathway for the administration of dexmedetomidine outside the PICU setting. Preliminary safety data, adverse effect profile, and efficacy outcomes are reviewed in pediatric-aged patients receiving a continuous dexmedetomidine infusion to treat pain and provide anxiolysis in various clinical scenarios outside the PICU setting.
| Materials and Methods | ▴Top |
This study was approved by the Institutional Review Board of Nationwide Children’s Hospital (Columbus, OH) and was conducted in compliance with the ethical standards of Nationwide Children’s Hospital for research involving human subjects, as well as with the Declaration of Helsinki. As a retrospective study, the need for individual written informed consent was waived.
Study cohort
The study cohort included patients ≤ 21 years of age who received a dexmedetomidine infusion for the management of acute or chronic pain outside the ICU setting. Patients receiving dexmedetomidine for another therapeutic effect or non-pain related conditions were excluded. We obtained a list of patients ≤ 21 years of age who received dexmedetomidine infusions from the records of the Acute Pain Service, the Pharmacy Database, and the EPIC Data Warehouse. These patients formed the cohort for this retrospective review.
Data collection
For each patient, demographic data including age, gender, weight, height, race, and ethnicity were collected. Additional information included the patient’s primary disease process, associated pain conditions, comorbid conditions, and the presence of end-organ dysfunction. Evaluation of laboratory data was carried out to evaluate end-organ function, including complete blood count, renal function, and hepatic function. Baseline and subsequent electrocardiogram (ECG) data were reviewed. Dexmedetomidine infusions were recorded, including the dose, bolus and infusion rates administered, duration of therapy, adverse effects, and any adjustments to the infusion rates. When the infusion rate was adjusted, the reason for the adjustment (inadequate analgesia, adverse effect, excessive sedation) was determined. Bradycardia and hypotension were defined as HR or BP less than the fifth percentile for age or a pause in the infusion related to HR or BP concerns. An adverse respiratory event was defined as an oxygen saturation (room air) less than 93%. For postoperative cases, if dexmedetomidine was administered intraoperatively, we determined the surgical procedure, the dose, and the duration of the intraoperative dexmedetomidine infusion. When available, additional subjective data from the daily progress notes of the Acute Pain Service were collected to determine efficacy and adverse effects.
Dexmedetomidine protocol and development
In November 2021, Nationwide Children’s Hospital Pain Management and Palliative Care team developed a hospital pathway for the administration of continuous dexmedetomidine infusions for analgesia, sedation, or anxiolysis outside the PICU setting. The protocol targeted the hematology, oncology, and bone marrow transplant inpatient units and specifically excluded patients receiving dexmedetomidine for sedation or anesthesia in the ICU setting. Attending physicians from the Acute Pain or Palliative Care Service who have received specialized training are able to prescribe this medication for the use of analgesia. Dexmedetomidine infusions are locked and administered via a patient-controlled analgesia (PCA) pump programed with a set range of doses for continuous infusions.
Eligible patients were those managed by the Acute Pain Service or Palliative Care Service with acute pain related to medical or surgical conditions (postoperative pain) or chronic pain including patients with chronic opioid requirements, palliative care patients, or patients with suspected opioid-induced hyperalgesia. Prior to the initiation of this pathway, the inpatient nursing staff was educated on dexmedetomidine’s dosing, indications, contraindications, adverse effects, patient monitoring requirements, and required documentation in the electronic medical record. Registered nurses administering dexmedetomidine also completed the intravenous opioid/sedatives/hypnotics class and mandatory modules through the hospital online learning system. After the completion of the modules, the nursing staff were required to take a post-test evaluation to receive credit for the training. Information regarding the use of dexmedetomidine for analgesia was added to the hospital’s pain management intranet website for reference.
These patients were managed by the inpatient pain service or palliative care service, which wrote the orders for the initiation of dexmedetomidine infusions, and provided consultations for patients receiving a continuous dexmedetomidine infusion. Dosing restrictions were included in this pathway. Dexmedetomidine was provided from the pharmacy in a concentration of 4 µg/mL, with the infusion rates ranging from 0.05 up to 0.6 µg/kg/h. The recommended starting infusion rate was 0.1 µg/kg/h. As needed, dexmedetomidine was titrated up in 0.05 µg/kg/h increments as tolerated, generally to a maximum infusion rate of 0.6 µg/kg/h. For hospice patients with an allow natural death (AND) order in place, the maximum infusion rate was 1 µg/kg/h. Ideal body weight was used for dosing in obese patients. Dose adjustments were made at a minimum of 30 min between doses to decrease the incidence of adverse physiologic effects. Infusions for ≥ 5 days were identified and required a slow taper of the continuous infusion or the use of clonidine as part of the weaning process to avoid withdrawal [7].
Specific monitoring guidelines were put in place to identify adverse effects and ensure patient safety. Patient assessment prior to the institution of a continuous infusion of dexmedetomidine included a standard patient history and physical examination. Patients with a prolonged QT interval, bradycardia, heart block, or other conduction delays were put on a precaution and considered for a baseline ECG before receiving treatment to evaluate entry into the study. Any allergy to dexmedetomidine was an absolute contraindication to receiving treatment. Patients receiving dexmedetomidine for sedative effects in the PICU or intraoperatively were also excluded from this protocol. The baseline assessment included respiratory rate, HR, BP, sedation levels, oxygen saturation, and pain scores. At the start of the infusion, HR, respiratory rate, and BP monitoring were required every 15 min for the first hour. This was decreased to every hour for 4 h, then every 2 h during the infusion. The same criteria were required for a dose escalation or if a change in patient status was identified. In addition, continuous pulse oximetry was required at all times along with a pain assessment every 4 h. The level of sedation and level of consciousness were assessed every 2 h while on the continuous infusion.
Statistical analysis and data presentation
Data were collected using REDCap and analyzed in Stata (version 18; StataCorp, College Station, TX). The analysis was primarily descriptive. Continuous variables were reported as medians and interquartile ranges (IQRs), as normality was assessed using the Shapiro-Wilk test and found to be non-normally distributed. Categorical variables were summarized as counts and column percentages.
| Results | ▴Top |
The study cohort included 26 patients ≤ 21 years, with a median age of 5 years (IQR: 2, 15) and a median weight of 18.9 kg (IQR: 12.7, 60). The majority (n = 18; 69.2%) of the patients were males (Table 1). Primary disease conditions in these patients were neuroblastoma (n = 12; 46.2%), followed by acute leukemia (n = 6; 23.1%), and sickle cell disease (n = 4; 15.4%). Ten patients underwent a procedure before the dexmedetomidine infusion. Intraoperative data, including anesthesia time and surgery times are outlined in Table 2. Seven of these patients were classified as American Society of Anesthesiologists (ASA) grade III. The median anesthesia time was 63.5 min (IQR: 27 - 70).
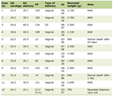 Click to view | Table 1. Demographic Data of the Retrospective Study Cohort |
 Click to view | Table 2. Dexmedetomidine Dosing Characteristics |
Dexmedetomidine infusion duration ranged from 1 to 28 days. The most common infusion durations were 1 day (n = 7; 26.9%) followed by 2 days (n = 5; 19.2%). All 26 patients experienced at least one dose adjustment. Most patients had both increases and decreases in the infusion rates (n = 16; 61.5%), while others experienced only an increase (n = 9; 34.6%) or only a decrease (n = 1; 3.8%). Five patients received a pre-infusion dexmedetomidine bolus, ranging from 2.5 to 26.2 µg, with a median dose of 4.2 µg
At least one adverse effect, which may or may not have been related to dexmedetomidine infusions, was experienced in 11 patients of the 26-patient cohort. The most common adverse effects were hypotension (n = 4) and delirium or hallucinations (n = 4). Sedation was reported in two patients while bradycardia occurred in one patient.
| Discussion | ▴Top |
Dexmedetomidine is an α2-adrenergic receptor agonist within the subclass of imidazole. It exerts a higher specificity ratio for α2 versus the α1-adrenergic receptor at 1,600:1 compared to clonidine, which has a 200:1 specificity ratio. Dexmedetomidine has a short half-life of 2 - 3 h and is traditionally used for short-term sedation in the ICU setting for mechanically ventilated patients [1]. In addition to its sedating effects, clinical actions include a dose-dependent analgesic effect by promoting parasympathetic activation and inhibition of substance P release within the dorsal horn of the spinal cord [5].
The hemodynamic and cardiovascular end-organ effects of dexmedetomidine generally following a biphasic pattern with an initial period of increased BP with bolus administration related to its peripheral effects on vascular α-adrenergic receptors followed by a slowing of HR and lowering of BP as a result of its central sympatholytic effects. In the absence of comorbid cardiovascular disease, these effects are generally well tolerated although caution and observation are suggested in patients with hypovolemia, comorbid myocardial dysfunction, pre-existing hypotension, or when administered concomitantly with other medication that possess negative chronotropic properties [7]. Dexmedetomidine also attenuates the body’s stress response, deeming it useful in many populations including surgical and palliative care patients [8]. In general, the effects on respiratory function are limited, especially when compared to other analgesic and sedative agents including opioids and benzodiazepines. A small decrease in tidal volume and mild hypercapnia that mimics respiratory changes during natural sleep have been reported with dexmedetomidine administration [8]. More significant respiratory depression are more commonly seen when dexmedetomidine is administered with other medications, at higher doses, or in patients with previously defined respiratory comorbidities [8].
Given its demonstrated safety profile and potential efficacy in various clinical scenarios, we developed a hospital-based protocol, as previously described, for its administration outside the PICU setting. The current cohort represents our initial clinical experience with that pathway. In general, the adverse effect profile was limited and easily corrected by pausing the infusion or decreasing the dose. Hemodynamic effects included hypotension in four patients and bradycardia in one. None of the hemodynamic effects required treatment with a vasoactive agent. There were no adverse respiratory effects or concerns identified in any of the patients in the current cohort.
Four patients within our cohort displayed signs of delirium or manifested hallucinations during the dexmedetomidine infusions. It is unclear if these issues could be attributed to the use of dexmedetomidine, or cofounding factors such as prolonged hospitalization, polypharmacy, or opioid intake. Dexmedetomidine has been shown to have a protective factor against delirium when compared to other sedating medications including benzodiazepines. The protective effect has been postulated to be related to the mimicking of a natural physiological sleep-wake cycle with dexmedetomidine sedation [1, 8]. Likewise, previous clinical studies have demonstrated a significant reduction of postoperative emergence delirium with dexmedetomidine use intraoperatively [9]. In the current cohort, most of the patients had significant comorbid conditions, both acute and chronic. As such, it is difficult to distinguish the true etiology of delirium, and it is feasible to postulate that the delirium may be from factors other than dexmedetomidine.
As a retrospective review, certain limitations must be recognized, most importantly the lack of randomization and the inability to precisely demonstrate a clinical benefit such as improved analgesia or a reduction in opioid requirements. We believe that adequately answering this question will require a prospective trial. With a retrospective design, it is also possible that specific adverse effects may be unrecognized or under-appreciated as a description of such events may not be clearly outlined in the medical record. Additionally, we recognize that our data are preliminary, given we present outcomes in a limited cohort size of 26 patients. The limited number also impacts the ability to definitely track the true incidence of adverse effects and their relationship to patient comorbid or clinical conditions.
Given the potential for respiratory or hemodynamic effects, the administration of dexmedetomidine in pediatric-aged patients is frequently restricted to the ICU setting. This was the case at our institution prior to the development of our clinical pathway for dexmedetomidine administration on the inpatient ward (see above). Previous reports have outlined the beneficial use of this medication in the palliative care setting outside the ICU and suggest its use with close monitoring for patients at risk of cardiovascular effects [10-12]. These reports have included an observational cohort study of nine pediatric palliative care patients on the inpatient floor with advanced heart disease, advanced malignancies, or post stem-cell transplantation. The cohort included patients experiencing escalating opioid requirements, opioid-induced hyperalgesia, or inadequate pain management. A significant reduction in pain scores and an overall decrease in opioid intake was reported without hemodynamic or adverse effects requiring intervention [11].
Additional valuable information regarding development of a pathway and educational process for nurses caring for patients receiving dexmedetomidine is outlined by Fannon et al [13]. The authors used a quality initiative to evaluate the process of implementing a new protocol with evidence-based interventions. The first aim included development and institution of a protocol guiding sedation with dexmedetomidine in spontaneously breathing pediatric patients with a native airway. The patients were monitored by the nursing staff in the PICU setting. The efficacy of their educational process was demonstrated by pre- and post-test results from 32 nurses over a 5-month period. More than 90% of the attending nurses had an increase in their knowledge and understanding of the safe use and monitoring for dexmedetomidine sedation in children.
Our preliminary experience provides further support demonstrating the safe use of dexmedetomidine infusions outside the ICU setting following a standardized protocol that includes patient selection, dosing parameters, monitoring, and training of physicians and nurses. Albeit rare, the most concerning adverse effects include the potential for hypotension and bradycardia. Within the dosing guidelines that we describe, the incidence of such adverse effects was low, and their clinical impact was limited. All adverse effects noted in the current cohort were successfully managed outside the ICU setting in conjunction with close monitoring. The administration of dexmedetomidine on the inpatient wards opens the use of this medication to a larger cohort of patients that can benefit from its physiologic properties. Additional clinical experience would be useful to clearly outline the role of dexmedetomidine in acute and chronic pain management as well as palliative care, define dosing strategies, and further demonstrate safety.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
As a retrospective study, the need for individual written informed consent was waived.
Author Contributions
Data acquisition, preparation of initial, interim, and final drafts (AW, CR); protocol development, review of final draft (SW); data acquisition, data analysis, final review of manuscript (SAYK); concept, writing, and review of all drafts (JDT).
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
Abbreviations
FDA: Food and Drug Administration; PICU: pediatric intensive care unit; AND: allow natural death; AE: adverse effect; MAC: monitored anesthesia care; AMP: adenosine monophosphate; ECG: electrocardiogram; PCA: patient-controlled analgesia
| References | ▴Top |
- Tobias JD. Dexmedetomidine: applications in pediatric critical care and pediatric anesthesiology. Pediatr Crit Care Med. 2007;8(2):115-131.
doi pubmed - Tobias JD, Gupta P, Naguib A, Yates AR. Dexmedetomidine: applications for the pediatric patient with congenital heart disease. Pediatr Cardiol. 2011;32(8):1075-1087.
doi pubmed - Correa-Sales C, Nacif-Coelho C, Reid K, Maze M. Inhibition of adenylate cyclase in the locus coeruleus mediates the hypnotic response to an alpha 2 agonist in the rat. J Pharmacol Exp Ther. 1992;263(3):1046-1049.
pubmed - Nacif-Coelho C, Correa-Sales C, Chang LL, Maze M. Perturbation of ion channel conductance alters the hypnotic response to the alpha 2-adrenergic agonist dexmedetomidine in the locus coeruleus of the rat. Anesthesiology. 1994;81(6):1527-1534.
doi pubmed - Correa-Sales C, Rabin BC, Maze M. A hypnotic response to dexmedetomidine, an alpha 2 agonist, is mediated in the locus coeruleus in rats. Anesthesiology. 1992;76(6):948-952.
doi pubmed - Lemus R, Jacobowski NL, Humphrey L, Tobias JD. Applications of dexmedetomidine in palliative and hospice care. J Pediatr Pharmacol Ther. 2022;27(7):587-594.
doi pubmed - Crabtree MF, Sargel CL, Cloyd CP, Tobias JD, Abdel-Rasoul M, Thompson RZ. Evaluation of an enteral clonidine taper following prolonged dexmedetomidine exposure in critically ill children. J Pediatr Intensive Care. 2022;11(4):327-334.
doi pubmed - Weerink MAS, Struys M, Hannivoort LN, Barends CRM, Absalom AR, Colin P. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin Pharmacokinet. 2017;56(8):893-913.
doi pubmed - Manning AN, Bezzo LK, Hobson JK, Zoeller JE, Brown CA, Henderson KJ. Dexmedetomidine dosing to prevent pediatric emergence delirium. AANA J. 2020;88(5):359-364.
pubmed - Coyne PJ, Wozencraft CP, Roberts SB, Bobb B, Smith TJ. Dexmedetomidine: exploring its potential role and dosing guideline for its use in intractable pain in the palliative care setting. J Pain Palliat Care Pharmacother. 2010;24(4):384-386.
doi pubmed - Burns J, Jackson K, Sheehy KA, Finkel JC, Quezado ZM. The use of dexmedetomidine in pediatric palliative care: a preliminary study. J Palliat Med. 2017;20(7):779-783.
doi pubmed - Hofherr ML, Abrahm JL, Rickerson E. Dexmedetomidine: a novel strategy for patients with intractable pain, opioid-induced hyperalgesia, or delirium at the end of life. J Palliat Med. 2020;23(11):1515-1517.
doi pubmed - Fannon S, Kisting MA, Anderson C. Implementation of a protocol: dexmedetomidine for use in long-term procedural sedation in non-intubated pediatric patients. J Pediatr Nurs. 2021;58:39-43.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
International Journal of Clinical Pediatrics is published by Elmer Press Inc.