| International Journal of Clinical Pediatrics, ISSN 1927-1255 print, 1927-1263 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Int J Clin Pediatr and Elmer Press Inc |
| Journal website https://www.theijcp.org |
Case Report
Volume 000, Number 000, December 2024, pages 000-000
Unveiling the Unexpected: A Rare Case of Silent Pediatric Cardiac Myxoma Mimicking Infection in an Eleven-Year-Old Girl
Sehrish Saleema, c, Mehwish Saleemb
aDepartment of Cardiothoracic Surgery, Shifa College of Medicine, Shifa International Hospital, H-8/4 Islamabad, Pakistan
bDepartment of Cardiothoracic Surgery, Shifa International Hospital, H-8/4 Islamabad, Pakistan
cCorresponding Author: Sehrish Saleem, Department of Cardiothoracic Surgery, Shifa College of Medicine, Shifa International Hospital, H-8/4 Islamabad, Pakistan
Manuscript submitted July 7, 2024, accepted December 11, 2024, published online December 21, 2024
Short title: An Atypical Presentation of Cardiac Myxoma
doi: https://doi.org/10.14740/ijcp543
| Abstract | ▴Top |
Pediatric cardiac myxoma is a rare tumor that commonly presents with cardiac or neurological symptoms. It can lead to serious complications like stroke, valvular obstructions, congestive heart failure, arrhythmias and peripheral embolism. We present a case of an 11-year-old girl with fever, right leg pain, swelling and redness. This presentation was initially attributed to an underlying infectious or rheumatological process but further testing revealed a large left atrial cardiac myxoma with peripheral embolism to the right common femoral artery. Review of literature reveals this being the second reported case of pediatric cardiac myxoma with systemic symptoms and non-neurological, non-cardiac presentation. Our patient underwent an uncomplicated excision of the mass along with femoral embolectomy. In this article, we highlighted the spectrum of clinical presentation of cardiac myxoma along with variety in its location, imaging features and the importance of considering intra-cardiac mass as a differential in pediatric population presenting with non-specific symptoms.
Keywords: Cardiac myxoma; Computed tomography angiography; Peripheral embolism; Small-vessel vasculitis; Cellulitis; Embolectomy; Femoral artery occlusion; Pediatric cardiac mass; Limb pain; Streptococcal pharyngitis
| Introduction | ▴Top |
Primary cardiac tumors are exceedingly rare in pediatric population with an incidence rate of 0.027% to 0.08% [1]. Cardiac myxoma is more common in adults [2, 3]. Most of these masses are benign in nature with rhabdomyoma being the most common type followed by benign teratoma and fibroma [1]. They most commonly present with cardiac symptoms such as dyspnea on exertion, orthopnea, syncope, palpitations, dizziness and neurologic symptoms such as transient ischemic attack, stroke or seizure primarily due to embolism [4]. Systemic embolism could also include the lungs leading to pulmonary embolism, the abdomen causing visceral hemorrhage and/or infarction and extremities leading to limb ischemia [2, 4]. Our case focuses on presentation of cardiac myxoma with peripheral embolism in absence of any cardiac or neurological manifestations. The rarity of such cases was highlighted after a meticulous literature review which reported only eight cases of pediatric cardiac myxoma with systemic and neurological symptoms and no cardiac manifestations [5-12] and only one case of peripheral embolism without concurrent cardiac and neurological symptoms [13] making our case the second reported case of such sort. We present a rare case of an adolescent girl who presented with right leg pain, fever and elevated inflammatory markers with no cardiac or neurological symptoms who was found to have cardiac myxoma. Given its rarity, this report highlights the spectrum of unique presentations of pediatric cardiac myxoma aiding the physicians in making a timely diagnosis.
| Case Report | ▴Top |
Investigations
An 11-year-old girl presented to the emergency department of our hospital with right leg pain and fever for 4 days. Both the symptoms started gradually. Fever ranged between 100 and 102 °F. It was constant and improved intermittently with ibuprofen. The right leg pain was initially mild and limited to the right hip and upper thigh region but later radiated to the entire leg. The pain was described as constant, deep seated and dull by the patient and graded 6/10 on pain intensity scale. It was not significantly relieved by ibuprofen. There was swelling and redness of the upper thigh and distal leg along with limb numbness but no associated rash was seen. Patient reported a history of bacterial pharyngitis a month ago which completely resolved with a prescribed 10-day course of amoxicillin-clavulanate. Her throat culture was positive for group B streptococcus, i.e. Streptococcus pyogenes. Patient denied any dizziness, syncope, visual symptoms, headaches, muscle weakness or family history of any significant disease including autoimmune diseases or cancer. Her only medication was ibuprofen for her fever and pain.
Physical examination on initial presentation showed an alert child who seemed to be in pain with a temperature of 102 °F, heart rate of 97/min, respiratory rate of 18/min, blood pressure of 120/85 mm Hg and 98% oxygen saturation on room air. On examination of her right leg, she reported tenderness to touch and severe pain with deep palpation in the hip, upper and mid-thigh region. Her right upper and mid-thigh were swollen with an increase of 2 cm in diameter as compared to the left leg. The affected limb had slight but diffusely erythematous skin. The right femoral artery and popliteal artery were weakly palpable, which at the time was attributed to limb edema. There was no joint effusion, joint pain or decreased range of motion. The remainder of the examination was unremarkable including a cardiac exam with normal S1, S2, no murmurs, rubs or gallops. The neurological exam showed normal sensory and motor examination with no focal neurological deficits. The cranial nerves were intact.
Diagnosis
With her recent history of strep throat, we kept soft tissue infections like cellulitis, erysipelas and few vasculitic entities versus septic emboli among the top of our differential list (Table 1).
 Click to view | Table 1. Differential Diagnosis |
The patient had elevated inflammatory markers along with leukocytosis (Tables 2 and 3). Two sets of blood cultures were performed due to concerns for sepsis but showed no growth. She was started on empirical antibiotic therapy with cefuroxime intravenous (IV) 100 mg/kg in three divided doses, vancomycin IV 60 mg/kg in three divided doses and acetaminophen IV 15 mg/kg every 6 h. Her fever resolved within 12 h of starting the medication. Chest X-ray, urinalysis, coagulation profile, liver function tests, renal function tests, and abdominal and renal ultrasound were unremarkable. Rheumatological workup included antistreptolysin O (ASO) titers, anti-DNAse B, cryoglobulins, lupus anticoagulant, antinuclear antibody (ANA), serum immunoglobulin A (IgA) levels, and complement levels, all of which were normal. Finally, duplex ultrasound of the right lower extremity was performed which showed complete occlusion of the right common femoral artery. Echocardiography was then performed to assess for intra-cardiac abnormalities and coronary vessel status due to concerns for vasculitis. Transthoracic echocardiography demonstrated a 4 × 5 cm pedunculated, hyperechogenic appearing mobile mass attached to the interatrial septum with a narrow stalk. Patient had normal biventricular function and ejection fraction. These specific features pointed towards cardiac myxoma, as the presence of a narrow stalk and attachment to interatrial septum is highly specific for the condition [14].
 Click to view | Table 2. Inflammatory Markers |
 Click to view | Table 3. Complete Blood Count With Differential Profile |
Cardiac magnetic resonance imaging (MRI) was suggested but was denied by the parents due to requirement of general anesthesia and longer imaging duration. Subsequently computed tomography angiography (CTA) of the chest and right lower limb was performed which confirmed the presence of an irregular left atrial mass and complete occlusion of the right common femoral artery (Figs. 1 and 2). The patient was diagnosed with left atrial myxoma complicated by embolization to right common femoral artery leading to acute limb ischemia.
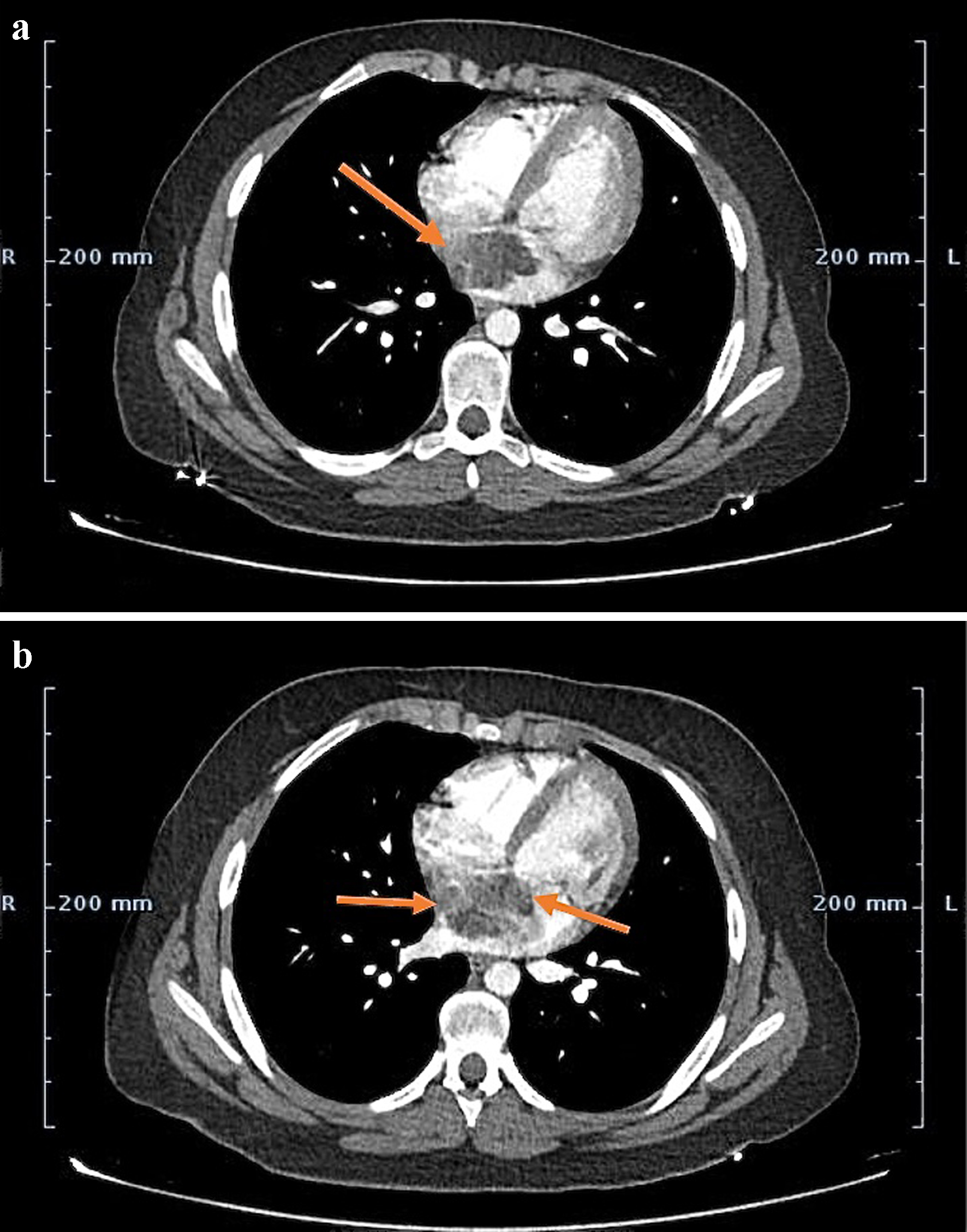 Click for large image | Figure 1. (a) Contrast-enhanced CT image at the level of aortic root demonstrating 4 × 5 cm heterogeneous appearing left atrial mass arising from the interatrial septum (arrow). (b) Mass demonstrating patchy enhancement secondary to intra-tumoral hemorrhage, necrosis and calcification (arrows). CT: computed tomography. |
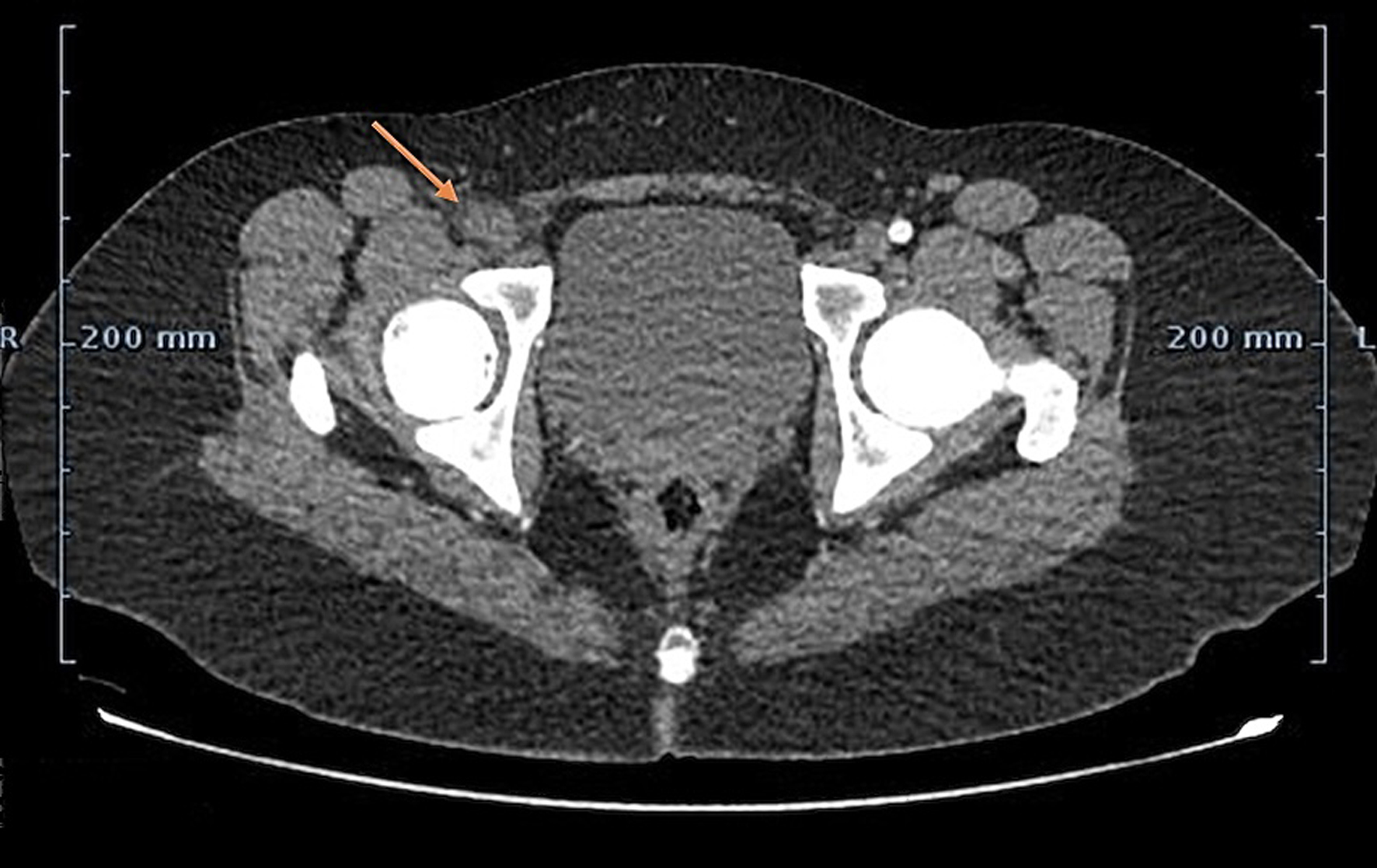 Click for large image | Figure 2. CTA of the right and left lower extremity demonstrating complete occlusion of the right common femoral artery (arrow). CTA:. |
Treatment
The patient was prepared for surgical excision of the left atrial mass and right femoral embolectomy. Pre-operative evaluation was normal. She was given injections cefuroxime IV 1.5 g and vancomycin IV 1 g prior to induction. The patient was stable on induction. The vascular surgeon first performed the right common femoral embolectomy uneventfully and established the flow into the right leg. There was a large left atrial mass attached to the interatrial septum along its postero-inferior margins. When hemodynamic monitoring lines were inserted, the patient was anesthetized with general endotracheal anesthesia and positioned correctly on the operating table. Routine cardiopulmonary bypass was instituted with bicaval venous cannulation and arterial return to the ascending aorta. Only antegrade cardioplegia setup was established. After application of the aortic cross clamp, cardioplegia was instilled into the aortic root which resulted in good electromechanical arrest. A routine right atriotomy was done and the septum primum was removed in its entirety along with the tumor. The gap created was then repaired with the patch of autologous pericardium with continuous 4/0 prolene sutures. Routine de-airing was done. The atriotomy was closed in usual fashion in two layers of continuous 4/0 prolene sutures. Clamp time was 35 min and the cardiopulmonary bypass (CPB) time was 45 min. When fully warm, the patient came off cardiopulmonary bypass in normal sinus rhythm uneventfully. Routine closure was performed with two chest drains. Histopathological report of the mass was consistent with cardiac myxoma. She was shifted to the pediatric intensive care unit (PICU) for postoperative recovery.
Follow-up and outcomes
Her recovery was uneventful and she was discharged 3 days later with instructions for follow-up. She was reported to be healthy without any symptom recurrence on follow-up at 1 and 3 months (Fig. 3).
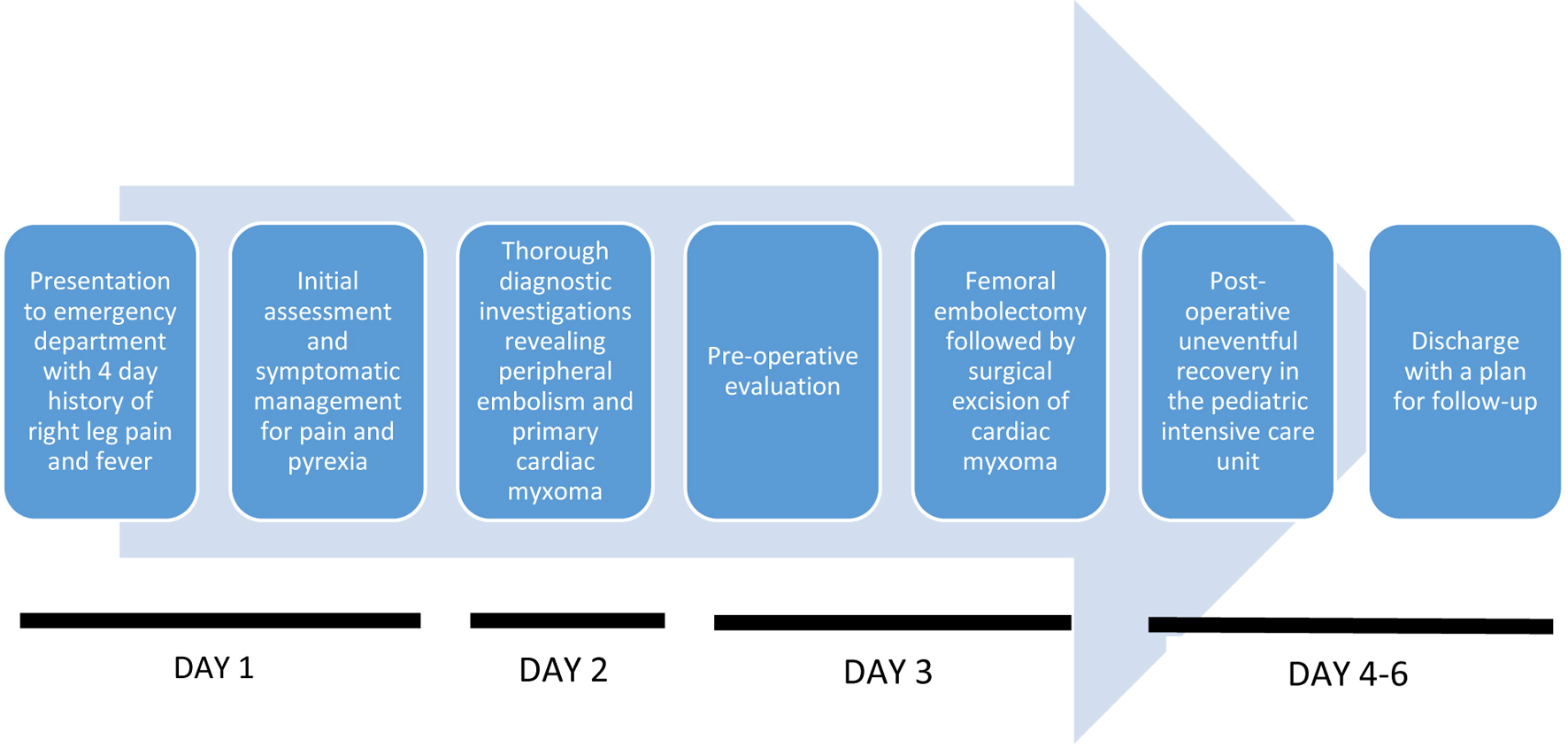 Click for large image | Figure 3. Timeline. |
| Discussion | ▴Top |
Incidence of cardiac myxoma in the pediatric population is quite rare. Most of cardiac myxomas occur sporadically with only 10% having a familial origin [15]. They are inherited as either autosomal dominant pattern or as a part of Carney complex which is due to a mutation in the PRKAR1A gene manifesting with periorificial lentigines, endocrine tumors (e.g., adrenal, pituitary glands, testicular tumors), and some non-endocrine tumors (e.g., cardiac myxoma, schwannomas) [16]. The tumor is well known to secrete pro-inflammatory cytokines mainly interleukin-6 which is responsible for the fever, weight loss and malaise associated with cardiac myxoma [5, 6]. Cardiac and neurological manifestations of cardiac myxoma secondary to flow obstruction or mass effect become apparent at an earlier stage. However, in context of peripheral embolism, given the often non-specific nature of the presenting symptoms, an early and accurate diagnosis of this potentially curable tumor can be relatively challenging.
The specific risk factors for embolization of cardiac myxomas include a large tumor size and irregular surface [17]. Chronic inflammation with tumor necrosis and vascularization also increases the likelihood of tumor embolization [18, 19]. A number of patients with cardiac myxomas have high inflammatory cytokine levels, which contribute to an increase in the production of platelets. This increase in mean platelet volume and platelet count may play a role in tumor-associated thromboembolic events [19]. On further exploration of the literature available, it was noted that polypoid-type cardiac myxomas with a friable or villous surface are more likely to cause embolism than solid round tumors and that these variants make up to 35% of all cardiac myxomas [20]. Furthermore, myxomas originating from the mitral valve may contribute more to embolization due to valve leaflets’ movement in comparison with myxomas of an atrial origin [20]. This further adds to the uniqueness of our case presented above, as the source of the embolus occluding the right common femoral artery was found to be a large left atrial myxoma. Tumor friability and irregular surfaces predispose them to turbulent blood flow and repetitive contact with the surrounding heart structures such as the valve leaflets, atrial and ventricular walls [18]. These structural and positional characteristics cause tumor fragmentation and detachment. Detached fragments enter the systemic circulation and lodge into the vasculature of distant organs, leading to complications like stroke, pulmonary embolism, limb ischemia, renal and splenic infarction, etc. [18, 19]. It is noted that arterial embolism associated with cardiac myxomas accounts for only 0.8-0.9% of all arterial limb embolism cases [21].
With respect to a timely diagnosis, echocardiography is considered a standard mode of investigation. Cardiac myxomas appear as intra-cavitary masses, commonly localized in the left atrium attached by a stalk to the fossa ovalis. They can be less commonly found in the right atrium, atrial free wall or mitral valve leaflet [22]. A more clinically sound approach to diagnosis of cardiac myxoma keeping in mind its wide array of extra-cardiac involvement will be to make use of cardiac CT and MRI in conjunction with an echocardiogram. Literature suggests that cardiac MRI is a more favorable imaging modality for cardiac masses compared to CT owing to its enhanced characterization of soft tissues, multi-planar images, an unrestricted field of view and above all, no requirement of the use of ionizing radiation which makes it ideal for pediatric patients along with echocardiography [23].
Due to the unique nature of such cases of cardiac myxomas, which often involve an unusual clinical presentation, the literature review carried out for this case report has its limitations. All information concluded above has been extracted from sources available in the PubMed database and therefore, cases provided on other external and unpublished sources may not have been taken into account.
Learning points
Our case above of an adolescent girl’s presentation with peripheral embolism in absence of cardiac or neurological symptoms underscores the atypical presentations of pediatric cardiac myxomas. Such rare cases often require a high index of suspicion and use of advanced imaging modalities such as echocardiography, CT, and MRI for accurate diagnosis. This report not only highlights the need for vigilance in diagnosing such tumors but also adds to the limited literature available on pediatric cardiac myxomas with atypical presentations. By focusing on the diversity of presentations of this tumor, our aim is to aid clinicians in making timely and accurate diagnoses and thus help improve patient outcome.
Acknowledgments
We acknowledge Dr. Mohammad Iqbal Khan and Dr. Khalid Rasheed for performing this surgery.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Written informed consent was obtained from the patient for publication of this case report, including the accompanying images.
Author Contributions
S. Saleem was involved in inpatient care, surgical assistance and out-patient follow-up. M. Saleem was involved in pediatric inpatient care. Both the authors contributed in writing, reviewing, editing and submission of the case report.
Data Availability
All the data in our report were obtained from patient’s hospitalization. Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
Abbreviations
ANA: antinuclear antibody; ASO: antistreptolysin O; cm: centimeter; CRP: C-reactive protein; CTA: computed tomography angiography; CT: computed tomography; DNAse B: deoxyribonuclease B; ESR: erythrocyte sedimentation rate; g/dL: grams per deciliter; IV: intravenous; IgA: immunoglobulin A; IL-6: interleukin-6; LDH: lactate dehydrogenase; mg/kg: milligrams per kilogram; mg/L: milligrams per liter; mm Hg: millimeters of mercury; mm/h: millimeters per hour; MRI: magnetic resonance imaging; ng/mL: nanograms per milliliter; pg/mL: picograms per milliliter; RBC: red blood cell; /UL: per unit liter; WBC: white blood cell
| References | ▴Top |
- Tzani A, Doulamis IP, Mylonas KS, Avgerinos DV, Nasioudis D. Cardiac tumors in pediatric patients: a systematic review. World J Pediatr Congenit Heart Surg. 2017;8(5):624-632.
doi pubmed - Shi L, Wu L, Fang H, Han B, Yang J, Ma X, Liu F, et al. Identification and clinical course of 166 pediatric cardiac tumors. Eur J Pediatr. 2017;176(2):253-260.
doi pubmed - Tao TY, Yahyavi-Firouz-Abadi N, Singh GK, Bhalla S. Pediatric cardiac tumors: clinical and imaging features. Radiographics. 2014;34(4):1031-1046.
doi pubmed - Gowda RM, Khan IA, Nair CK, Mehta NJ, Vasavada BC, Sacchi TJ. Cardiac papillary fibroelastoma: a comprehensive analysis of 725 cases. Am Heart J. 2003;146(3):404-410.
doi pubmed - Saji T, Matsuo N, Shiono N, Yokomuro H, Watanabe Y, Takanashi Y, Komatsu H. [Serum/tissue interleukin-6 concentrations and constitutional abnormalities in 4 patients with cardiac myxoma]. Kokyu To Junkan. 1993;41(9):891-895.
pubmed - Hovels-Gurich HH, Seghaye MC, Amo-Takyi BK, Hugel W, Duchateau J, von Bernuth G. Cardiac myxoma in a 6-year-old child—constitutional symptoms mimicking rheumatic disease and the role of interleukin-6. Acta Paediatr. 1999;88(7):786-788.
doi pubmed - Goldberg HP, Glenn F, Dotter CT, Steinberg I. Myxoma of the left atrium; diagnosis made during life with operative and post-mortem findings. Circulation. 1952;6(5):762-767.
doi pubmed - Park JM, Garcia RR, Patrick JK, Waagner D, Anuras S. Right atrial myxoma with a nonembolic intestinal manifestation. Pediatr Cardiol. 1990;11(3):164-166.
doi pubmed - Shiraishi I, Yamagishi M, Kato R, Okumura Y, Sato H, Tanaka T, Hamaoka K. A case in a child of giant left-atrial myxoma associated with recurrent high fever and myxoma cells expressing interleukin-6. Eur J Pediatr. 2006;165(5):346-347.
doi pubmed - Patel R, Lynn KC. Masquerading myxoma. Am J Med Sci. 2009;338(2):161-163.
doi pubmed - Kaminsky ME, Ehlers KH, Engle MA, Klein AA, Levin AR, Subramanian VA. Atrial myxoma mimicking a collagen disorder. Chest. 1979;75(1):93-95.
doi pubmed - Margarint I, Sorescu A, Popescu M, Robu M, Untaru O, Filip C. A unique case of a gigantic left ventricular myxoma resulting in embolic acute lower limb ischemia in a pediatric patient. J Clin Med. 2024;13(8):2189.
doi pubmed - Macias E, Nieman E, Yomogida K, Petrucci O, Javidan C, Baszis K, Anwar S. Rare presentation of an atrial myxoma in an adolescent patient: a case report and literature review. BMC Pediatr. 2018;18(1):373.
doi pubmed - Araoz PA, Mulvagh SL, Tazelaar HD, Julsrud PR, Breen JF. CT and MR imaging of benign primary cardiac neoplasms with echocardiographic correlation. Radiographics. 2000;20(5):1303-1319.
doi pubmed - Molina JE, Edwards JE, Ward HB. Primary cardiac tumors: experience at the University of Minnesota. Thorac Cardiovasc Surg. 1990;38(Suppl 2):183-191.
doi pubmed - Vindhyal MR, Elshimy G, Haq N, Elhomsy G. Carney complex. In: StatPearls. 2024.
pubmed - Kalcik M, Bayam E, Guner A, Kup A, Kalkan S, Yesin M, Gursoy MO, et al. Evaluation of the potential predictors of embolism in patients with left atrial myxoma. Echocardiography. 2019;36(5):837-843.
doi pubmed - Liu Y, Wang J, Guo L, Ping L. Risk factors of embolism for the cardiac myxoma patients: a systematic review and metanalysis. BMC Cardiovasc Disord. 2020;20(1):348.
doi pubmed - He DK, Zhang YF, Liang Y, Ye SX, Wang C, Kang B, Wang ZN. Risk factors for embolism in cardiac myxoma: a retrospective analysis. Med Sci Monit. 2015;21:1146-1154.
doi pubmed - Keeling IM, Oberwalder P, Anelli-Monti M, Schuchlenz H, Demel U, Tilz GP, Rehak P, et al. Cardiac myxomas: 24 years of experience in 49 patients. Eur J Cardiothorac Surg. 2002;22(6):971-977.
doi pubmed - Zhou H, Yin Y, Sun Z. Clinical characteristics of acute lower extremity ischemia due to left atrial myxoma: a rare case report with review of literature. Heart Surg Forum. 2023;26(3):E292-E302.
doi pubmed - Parato VM, Nocco S, Alunni G, Becherini F, Conti S, Cucchini U, Di Giannuario G, et al. Imaging of cardiac masses: an updated overview. J Cardiovasc Echogr. 2022;32(2):65-75.
doi pubmed - Kassop D, Donovan MS, Cheezum MK, Nguyen BT, Gambill NB, Blankstein R, Villines TC. Cardiac masses on cardiac CT: a review. Curr Cardiovasc Imaging Rep. 2014;7(8):9281.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
International Journal of Clinical Pediatrics is published by Elmer Press Inc.